Figure 1.
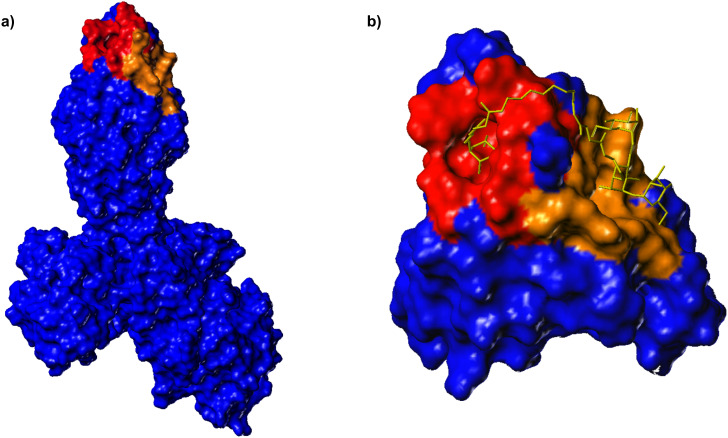
a) Connolly surface of FimH in complex with FimC [8]. The CRD known from X-ray structures at the tip of FimH is coloured in red and can accommodate one α-D-mannosyl residue. A second hypothetical carbohydrate binding region on the protein, as suggested by modeling studies [16], is coloured in brown and represents a more extended area on the protein. b) Docking studies were used to estimate the length of a linker that is required to bridge the putative two binding sites.