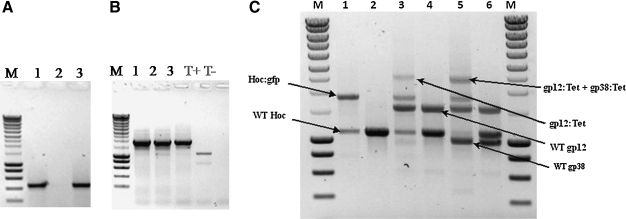
Figure 4.
Rho* expression inhibits phage replication, preserves the phage genome within the host, and allows high-efficiency, stable homologous recombination of phage's genes. DK8 E. coli host cells overexpressing a nonfunctional variant of the rho gene (Rho*) were infected with wild-type T4 phages. Host cells then were collected 3 hours and 96 hours following phage infections, lysed, and the total cellular DNA subjected to PCR analysis directed toward the T4-specific gp38 gene followed by gel electrophoresis. Figure 4A shows that, in spite of the near complete absence of host lysis, PCR analyses revealed the presence at high frequency of T4 gp38 genes in host cells 3 hours (lane 1) and 96 hours (lane 3) following infection, while failing to give a product from noninfected host cells (lane 2). A hoc-GFP construct was electroporated into Rho* overexpressing DK8 cells harboring the cloned Red-recombinase system together with a “deactivated” wild-type T4 genome. Following the induction of Red-mediated homologous recombination, parts of the cells were used for PCR analysis directed toward the hoc-GFP construct (Figure 4B, lane 1). The remainder of the cells were left to grow for 12 hours and the T4 lytic cycle restored (see text). An aliquot of the ensuing phage progeny was used for PCR analysis of the hoc genetic locus (Figure 4B, lane 2), and the remainder were used to infect standard DK8 host cells. The second-generation progeny were analyzed by PCR as above (Figure 4B, lane 3). PCR analysis of the hoc locus in the first-generation progeny (lane 2) revealed a remarkably high apparent recombination rate. The strong PCR band corresponding to the hoc-GFP construct with the concurrent presence of a weak wild-type hoc signal (cf: lane T+) indicated that a very significant proportion of the first-generation progeny appeared to carry the recombinant construct instead of the wild-type gene. This was confirmed by the analytical results obtained from the second-generation progeny (lane 3), in which the recombinant construct was overwhelmingly present and the wild-type hoc sequence hardly detectable. (T+: wild-type PCR hoc signal from DK8 hosts harboring a “deactivated T4 genome; T-: noninfected control DK8 cells). Simultaneous recombinations at multiple loci within the phage's genome were efficiently achieved (Figure 4C, lanes 1, 3, and 5) following concurrent electroporation of hoc-GFP and constructs consisting of the gp12 and gp38 genes fused to a tetracycline resistance encoding insert (gp12:Tet and gp38:Tet, respectively), as demonstrated by postrecombination PCR analyses of the ensuing T4 progeny shown in Figure 4C (M: Molecular weights marker; lane 1: Hoc:GFP insert into the T4 genome; lane 2: WT hoc control; lane 3: Hoc:GFP + gp12:Tet inserts into the T4 genome; lane 4: WT hoc + WT gp12 control; lane 5: Hoc:GFP + gp12:Tet + gp38:Tet inserts into the T4 genome; lane 6: WT hoc + WT gp12 + WT gp38 control).