Table 2.
In vitro activity of the (Z)-methyl 2-(2-oxo-2H-benzo[b][1,4]oxazin-3(4H)ylidene)acetatesa
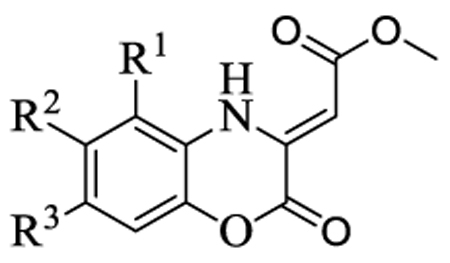 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | IC50b (µM) |
Ki (µM) |
Ki’ (µM) |
MICc (µg/ml) |
| 1 | H | H | H | 10.0±1.0 | 9.1±1.2 | 67.0±7.9 | 0.64 |
| 2 | Me | H | H | 24.1±1.8 | 25 | ||
| 3 | H | Me | H | 23.1±1.0 | >100 | ||
| 4 | H | F | H | 27.0±3.0 | 11.5±1.5 | 10.1±0.9 | 0.63 |
| 5 | H | Cl | H | 46.3±3.5 | 22.5±1.1 | 18.5±2.6 | 5 |
| 6 | H | NO2 | H | 28.2±4.4 | 50 | ||
| 7 | H | EtSO2 | H | 17.9±3.0 | >100 | ||
| 8 | H | H | Me | 18.2±2.8 | 100 | ||
| 9 | H | H | F | 30.0±3.7 | 0.63 | ||
| 10 | H | H | Cl | 35.7±4.8 | 0.63 | ||
| 11 | H | H | NO2 | 20.3±1.8 | >100 | ||
| 12 | 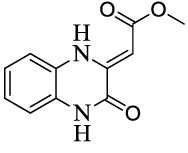 |
>122 | >100 | ||||
| 13 | 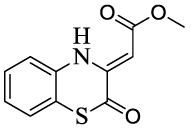 |
>140 | >100 | ||||
MenB concentration was 150 nM.
Compound concentration giving 50% inhibition of enzyme activity.
Compound concentration giving 90% inhibition of M. tuberculosis H37Rv growth.
