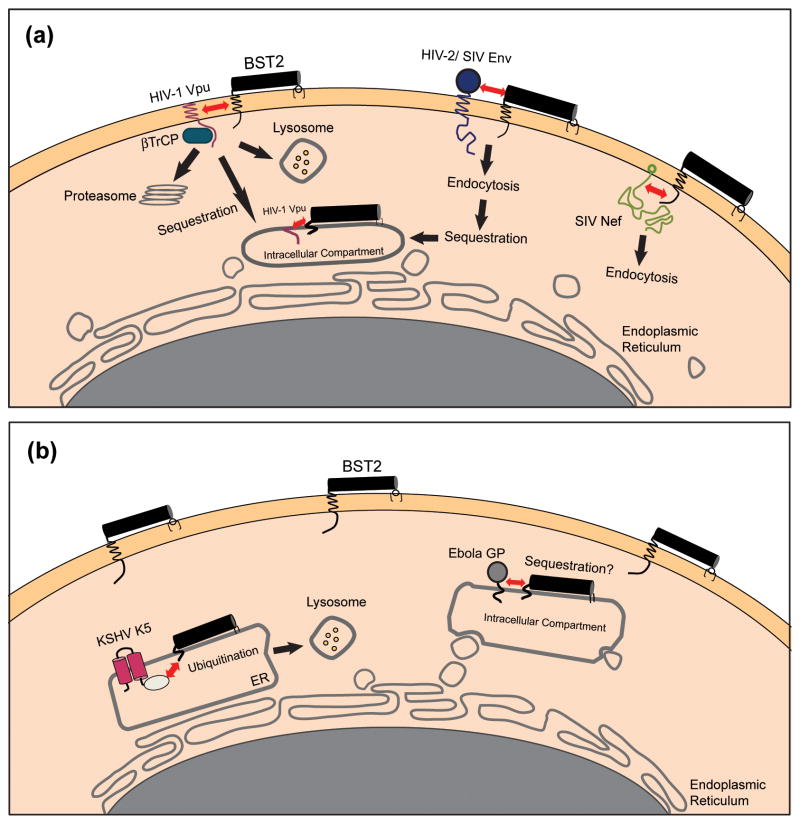
Figure 3. Viral antagonists of BST-2 and their potential mechanisms of action.
(a) Lentiviral antagonists. The HIV-1 Vpu protein associates with BST-2 through transmembrane domain interactions and recruits βTrCP, leading to the degradation of BST-2 within lysosomes and/or proteasomes. Alternatively, Vpu might simply trap BST-2 within the endosomal system. In either scenario, the concentration of BST-2 is decreased at the plasma membrane, its site of action as a tetherin. The SIV Nef protein interacts with the cytoplasmic domain of BST-2 and mediates downregulation from the cell surface, most likely via endocytosis. The HIV-2 Env protein downregulates and sequesters BST-2 within the trans-Golgi network (TGN) by a mechanism that depends on physical interactions between the two proteins and a conserved GYXXφ motif in the cytoplasmic tail of gp41. (b) Non-lentiviral antagonists. The Ebola GP physically associates with, and possibly sequesters, BST-2 within intracellular compartments. Alternatively, Ebola GP might directly interfere with the restrictive activity of BST-2 or it might direct viral assembly away from membrane domains containing BST-2. The KSHV K5 protein mediates the ubiquitination of BST-2, which induces degradation of the protein within proteasomes and/or lysosomes. The interactions between BST-2 and the lentiviral antagonist proteins Vpu, Nef, and Env are shown here as occurring at the plasma membrane, but they could occur alternatively or additionally within the endoplasmic reticulum (ER), the TGN, or recycling endosomes.