Table 1.
Comparison of the conformational dynamics induced by different VDR modulators between VDR-LBD and full length VDR/RXR heterodimer. The values represent the average difference in percentage of deuterium incorporation of apo VDR-LBD and apo full length VDR/RXR in presence of difference VDR modulators across all H/D exchange time points (%). The regions with statistically significant differential deuteration level were colored. Statistical summary from a two-way ANOVA between apo and ligand bound data, P< 0.001. The value in parentheses represents the charge state of the peptide ion. Exchange kinetics for 41 different regions of the receptor LBD were measured and shown in Figure S2. See also Figure S1.
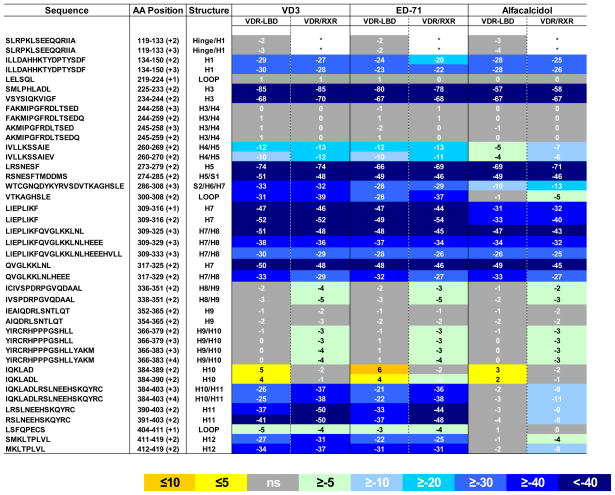 |
This exact peptide representing the hinge/H1 region (residues 119–133), the very N-terminus of VDR-LBD, was not identified in the HDX study of full length VDR/RXR heterodimer, as a different peptide is generated covering this region in the full length protein.
