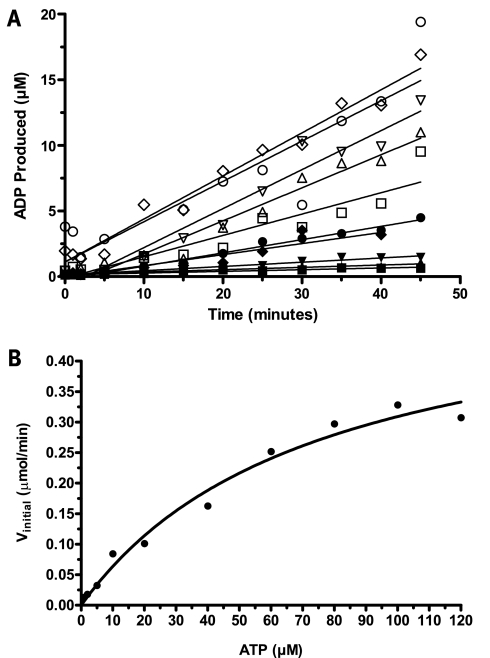
FIG. 4.
The ATP Km for a target kinase was determined by measuring Vinitial values for a range of ATP concentrations using multiple standard curves. (A) Kinase reactions were performed in 10 μl (2.5 μl of kinase, 2.5 μl of substrate, and 5 μl of ATP, all in CK buffer) for a number of fixed time points (up to 45 min), with varying ATP concentrations (up to 120 μM). Kinase reactions were stopped by adding 10 μl of the ADP Detection Mixture 1 (containing two times the EC85 Ab1 concentrations), and mP values were measured after a 1-h equilibration. Polarization values were converted to the amount of ADP produced using a standard curve for each ATP concentration: 1 μM ATP (▪), 2 μM ATP (▴), 5 μM ATP (▾), 10 μM ATP (♦), 20 μM ATP (•), 40 μM ATP (□), 60 μM ATP (▵), 80 μM ATP (▿), 100 μM ATP (◊), 120 μ ATP (○). (B) A representative Michaelis-Menten plot of the initial rates versus ATP concentration; in replicate experiments, the Km value was determined to be 72 6 3.3 μM (standard error of the mean).