Table 2.
Representative ring expansions of cycloadducts.
| entry | cycloadduct | conditionsa | product/yield (isolated) | dienophilic equivalent |
|---|---|---|---|---|
| 1 | A |
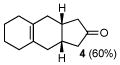 4 |
10 |
|
| 2 |
 3f |
B |
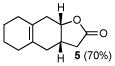 5 |
11 |
| 3 | C |
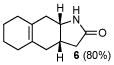 6 |
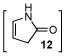 12 |
|
| 4 | A |
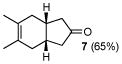 7 |
||
| 5 |
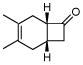 3h |
B |
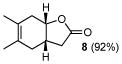 8 |
|
| 6 | C |
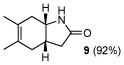 9 |
||
Key. A: Trimethylsulfoxonium iodide, NaH, DMF, then LiI, THF; B: CF3CH2OH, H2O2.; C: O-Mesitylenesulfonyl-hydroxylamine, CH2Cl2, 0°C, then Al2O3, PhH/MeOH(3:1).
