Abstract
Extracellular DNA (eDNA) is a structural component of the polymeric matrix of biofilms from different species. Different mechanisms for DNA release have been proposed including lysis of cells, lysis of DNA-containing vesicles, and DNA secretion. Here, a genome-wide screen of 3985 non-lethal mutations was performed to identify genes whose deletion alters eDNA release in Escherichia coli. Deleting nlpI, yfeC, and rna increased eDNA from planktonic cultures while deleting hns and rfaD decreased eDNA production. The lipoprotein NlpI negatively affects eDNA since the overexpression of nlpI decreases eDNA 16 fold while deleting nlpI increases eDNA 3 fold. The global regulator H-NS is required for eDNA production since DNA was not detected for the hns mutant and production of H-NS restored eDNA production to wild-type levels. Therefore our results suggest that secretion may play a role in eDNA release in E. coli since the effect of the hns deletion on cell lysis (slight decrease) and membrane vesicles (3 fold increase) does not account for the reduction in eDNA.
Keywords: extracellular DNA, H-NS, NlpI
INTRODUCTION
Bacteria accumulate at interfaces forming biofilms, communities of cells embedded in a self-produced polymeric matrix. The matrix constitutes about 90% of the mass of the biofilm and mainly consists of extracellular polysaccharides, proteins, lipids, and nucleic acids [1]. The extracellular DNA (eDNA) component of the biofilm matrix has been found in many Gram positive [2,3,4,5] and Gram negative bacteria [6,7,8] and serves many roles in different bacteria; eDNA is required for initial attachment to a surface [2,5,9], has a structural role connecting the cells in the biofilm [2,7,10,11], works as a nutrient source [12,13], contributes to cation gradients, induces antibiotic resistance, and promotes its own release via cell lysis by destabilizing membranes through cation chelation [14]. eDNA also facilitates horizontal gene transfer and DNA uptake [15].
eDNA also works as an interconnecting material for planktonic cells for Pseudomonas aeruginosa where microscopic observation and DNase I treatment indicate that planktonic cells are connected by eDNA-forming clumps [11]. Another example is the marine photosynthetic bacterium Rhodovulum sulfidophilum which forms aggregated communities of cells called flocs [16] joined by extracellular DNA and RNA [17].
The origin of eDNA is not clear since some reports indicate that eDNA is similar to genomic DNA (gDNA) [9,11,16] but other studies revealed, by comparing eDNA and gDNA through random amplification, that they are different [10,17]. The most common mechanism of eDNA release is cell lysis [9,11,15,18]. However, it has been proposed that membrane vesicles (MVs) released from the outer membrane also participate in eDNA production [6] since when MVs are opened, extracellular DNA and enzymes that promote lysis are liberated [19]. Some bacteria produce eDNA by direct secretion from intact cells such as Neisseria gonorrhoeae that produces eDNA via type IV secretion system [20].
eDNA release has been related to quorum-sensing in Streptococcus pneumoniae via the competence-stimulating peptide (CSP) [15] and in P. aeruginosa via acylhomoserine lactones (AHLs) and PQS signaling [11]. We also have reported that eDNA levels are inversely related to c-di-GMP in P. aeruginosa [21] as regulated by tyrosine phosphorylation regulator TpbA [22].
Here, we sought to identify the genes controlling the release of eDNA in E. coli [23] in order to understand better the nature of its release from this strain; to date the mechanism of DNA release in this best-studied strain has not been addressed. We screened the entire Keio collection of 3985 E. coli K-12 BW25113 single gene knock-out mutants for eDNA using a fluorescence dye to stain the DNA present in the supernatant of cultures grown quiescently in minimal media in microtiter plates. The mutations altering eDNA are related to general cellular processes such as DNA replication, transcription, translation, nutrient transport and metabolism, and cell envelope. Specifically, the nlpI, yfeC, and rna mutants increased eDNA and the hns and rfaD mutants decreased eDNA production. The role of cell lysis and MVs on eDNA with the nlpI and hns mutants was also investigated; these results suggest DNA is secreted by a process controlled by H-NS.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions
The E. coli strains and plasmids used in this study are listed in Table 1. We used the 3985 E. coli K-12 BW25113 single gene knock-out mutants from the Keio collection [24] for the eDNA screening and the ASKA library [25] for overexpression of specific genes. Cultures were made in Luria-Bertani (LB) [26]. Kanamycin (50 μg/mL) was used for pre-culturing the knock-out mutants, carbenicillin (100 μg/mL) was used for pLP170, and chloramphenicol (30 μg/mL) was used for selecting plasmid pCA24N and its derivatives. All experiments were conducted at 37°C.
Table 1.
E. coli strains and plasmids used in this study.
| Strains and plasmids | Genotype/relevant characteristicsa | Source |
|---|---|---|
| Strains | ||
| BW25113 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) LAM− rhp-1 Δ (rhaD- rhaB)568 hsdR514; parental strain for the Keio collection. | Yale CGSG Stock Center |
| BW25113 hns | BW25113 Δhns746::kan KmR | [24] |
| BW25113 nlpI | BW25113 ΔnlpI775::kan KmR | [24] |
| BW25113 rfaD | BW25113 ΔrfaD731::kan KmR | [24] |
| BW25113 rna | BW25113 Δrna749::kan KmR | [24] |
| BW25113 yfeC | BW25113 ΔyfeC732::kan KmR | [24] |
| BW25113 hha | BW25113 Δhha745::kan KmR | [24] |
| BW25113 hha hns | BW25113 Δhha845 Δhns746::kan KmR | [48] |
| Plasmids | ||
| pCA24N | lacIq, CmR | [25] |
| pCA24N-hns | pCA24N PT5-lac::hns CmR | [25] |
| pCA24N-nlpI | pCA24N PT5-lac::nlpI CmR | [25] |
| pLP170 | promoterless lacZ fusion vector CbR | [49] |
KmR, CmR, CbR are kanamycin, chloramphenicol, and carbenicillin resistance, respectively.
eDNA screening
The mutants from the Keio collection were transferred from glycerol stocks, using a 96 pin replicator (Boekel Scientific, Feasterville, PA), to 96-well polystyrene plates (Corning, Lowell, MA) containing 300 μL of AB medium [27] supplemented with 0.2 % glucose and 0.4% casamino acids and were incubated for 24 h without shaking. AB medium [11] was used for the screening since LB medium interfered with the fluorescence dye used for detecting eDNA. Cell density was measured at 620 nm with a Sunrise microplate reader (Tecan, Salzburg, Austria), and the 96-well plates were centrifuged at 4150 rpm for 10 min using a AccuSpin 3R centrifuge (Fisher Scientific Co, Pittsburgh, PA). The amount of DNA in 100 μL of supernatant was determined with Quant-iT PicoGreen dsDNA kit (Molecular Probes, Eugene, OR) using a Spectra Max Germini EM fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) with an excitation wavelength of 480 nm and emission wavelength of 520 nm. The amount of DNA was normalized by the cell density, and the mutants that significantly altered eDNA were screened again against the wild-type BW25113 using at least three independent colonies of each strain.
Quantitative polymerase chain reaction (qPCR)
eDNA was purified as described previously [21] from cells cultured for 24 h in LB with shaking at 250 rpm starting from an initial turbidity at 600 nm of 0.05. The culture (1 mL) was centrifuged at 13 krpm for 10 min, and the supernatant was used for eDNA purification using phenol:chloroform:isoamyl alcohol (25:24:1) extraction and sodium acetate and isopropanol precipitation. To normalize the eDNA by the total amount of DNA in the cells and in the supernatant, one mL of culture was sonicated for 45 s at 10 W (60 Sonic Dismembrator, Fisher Scientific Co, Pittsburgh, PA) and centrifuged at 13 krpm for 10 min; the supernatant was used for total DNA purification. The purified eDNA and total DNA from at least two independent cultures of each strain was quantified by qPCR using the StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA) and the SuperScriptTM III Platinum® SYBR® Green One-Step qRT-PCR Kit (Invitrogen, Carlsbad, CA) with primers for the reference gene purA (purA-f 5′GGGCCTGCTTATGAAGATAAAGT-3′ and purA-r 5′-CAACCACCATAGAAGTCAGGT-3′).
Cell lysis assay
BW25113 and the hns and nlpI mutants expressing lacZ from pLP170 were cultured into 25 mL of LB medium starting from a cell density of 0.05 at 600 nm for 24 h, 250 rpm. The β-galactosidase activity of the culture supernatants was normalized by the total β-galactosidase activity of the sonicated cultures and used to evaluate cell lysis as described previously [28].
Membrane vesicles
MVs were purified as described previously [29], with some modifications. BW25113, nlpI, and hns cultures in LB with an initial turbidity at 600 nm of 0.03 were grown for 14 h then centrifuged at 6000 g for 10 min at 4°C. The supernatants were filtered through a 0.22 μm vacuum filter (Millipore Co., Billerica, MA) and concentrated by ultrafiltration using a 100 kDa cut-off Diaflo membrane (Amicon Co., Lexington, MA) in a stirred ultrafiltration cell (model 8200, Amicon Co., Lexington, MA). The concentrated supernatants were ultracentrifuged at 30 krpm for 1 h at 4°C in a SW41 Ti rotor (154,100 g) using the Beckman L8-M ultracentrifuge (Beckman Coulter Inc., Brea, CA); the supernatants were decanted and the precipitated membrane vesicles were resuspended with 50 mM HEPES pH 6.8 buffer. The amount of MVs was determined using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA).
RESULTS
Screening of the genes involved in eDNA production
To identify genes involved in E. coli eDNA production, we screened 3985 nonessential gene knock-out mutants of the Keio Collection [24]. Since the mutants were grown quiescently in microtiter plates, the eDNA detected in the screening was produced from both biofilm and planktonic cells. The screening was performed based on the fluorescence of Quant-iT PicoGreen reagent upon binding to double stranded DNA (dsDNA); the sensitivity with the conditions used for the assay was 0.004 ng dsDNA/μL. After two rounds of screening, four mutants that increase eDNA and 31 mutants that decrease eDNA more than 2.5 fold were identified (Table 2). These genes encode proteins mainly located in the cytoplasm that are related to different cellular processes including the synthesis of components of the cell envelope such as lipopolysaccharide (LPS).
Table 2.
E. coli BW25113 genes whose mutations altered eDNA as detected by Quant-iT PicoGreen. The BW25113 value corresponds to the average of 31 independent colonies and for each mutant at least 3 colonies were assayed. For all listed mutants, differences in eDNA compared to the wild-type are significant based on a Student’s T test (P < 0.05). Locations are from [50] and function are from [51,52].
| Strain | OD620 | ng DNA μL−1 OD620−1 | Location | Fold | Function |
|---|---|---|---|---|---|
| BW25113 | 1.1 ± 0.2 | 0.53 ± 0.07 | - | 1 | - |
| Replication, recombination and repair | |||||
| priA | 0.62 ± 0.03 | 0.17 ± 0.01 | C | −2.9 | PriA participates in DNA replication |
| Transcription and translation | |||||
| rna | 0.9 ± 0.2 | 2.2 ± 0.6 | P | 4.4 | RNase I, cleaves phosphodiester bonds in RNA |
| hns | 0.67 ± 0.05 | 0.17 ± 0.02 | C | −2.9 | DNA-binding global regulator H-NS |
| pnp | 1.1 ± 0.1 | 0.16 ± 0.01 | C | −3.1 | PNPase, involved in general mRNA degradation |
| Posttranslational modification, protein turnover, chaperones | |||||
| groL | 1.2 ± 0.3 | 0.15 ± 0.01 | C | −3.3 | Chaperone Hsp60 |
| sspA | 1.1 ± 0.2 | 0.16 ± 0.01 | C | −3.1 | Protein essential for cell survival under acid-stress |
| Metabolism | |||||
| cyaA | 0.99 ± 0.07 | 0.10 ± 0.01 | C | −5.0 | Adenylate cyclase CyaA catalyzes the synthesis of cyclic AMP |
| aspC | 0.76 ± 0.02 | 0.11 ± 0.01 | C | −4.5 | Aspartate aminotransferase |
| gmhB | 0.85 ± 0.08 | 0.13 ± 0.02 | C | −3.8 | D, D-heptose 1,7-bisphosphate phosphatase |
| btuB | 1.0 ± 0.3 | 0.15 ± 0.06 | OM | −3.3 | Receptor for transport of vitamin B12, E colicins, and phages BF23 and C1 |
| moaA | 0.7 ± 0.04 | 0.15 ± 0.03 | C | −3.3 | Protein that participates in the synthesis of molybdopterin guanine dinucleotide |
| moaC | 0.59 ± 0.04 | 0.19 ± 0.03 | C | −2.6 | Protein that participates in the MPT biosynthesis |
| moaE | 0.8 ± 0.3 | 0.13 ± 0.03 | C | −3.8 | MPT synthase |
| mog | 0.82 ± 0.07 | 0.21 ± 0.01 | C | −2.8 | Protein that participates in the MPT biosynthesis |
| menD | 1.16 ± 0.05 | 0.14 ± 0.02 | C | −3.6 | Protein that participates in menaquinone biosynthesis |
| menE | 1.14 ± 0.08 | 0.11 ± 0.01 | C | −4.5 | Protein that participates in menaquinone biosynthesis |
| nudB | 1.1 ± 0.2 | 0.20 ± 0.04 | C | −2.5 | Protein that participates in the early steps in folate synthesis |
| Inorganic ion transport | |||||
| pstA | 1.7 ± 0.07 | 0.12 ± 0.01 | IM | −4.2 | Part of the ATP-dependent phosphate uptake system PstABCS |
| pstS | 1.0 ± 0.3 | 0.13 ± 0.04 | P | −3.8 | Part of the ATP-dependent phosphate uptake system PstABCS |
| phoU | 1.1 ± 0.3 | 0.15 ± 0.04 | C | −3.3 | Negative regulator of pho regulon (phosphate transport system) |
| modC | 0.6 ± 0.2 | 0.18 ± 0.01 | C | −2.8 | ATP-binding component of the molybdate ABC transporter |
| Cell envelope | |||||
| lpcA | 0.65 ± 0.08 | 0.19 ± 0.02 | C | −2.6 | Catalyzes the first step in the synthesis of core lipopolysaccharide (LPS) |
| rfaD | 0.63 ± 0.02 | 0.16 ± 0.03 | C | −3.1 | Involved in the synthesis of the precursor of core LPS |
| rfaE | 0.61 ± 0.05 | 0.16 ± 0.01 | C | −3.1 | Involved in the synthesis of the precursor of core LPS |
| rfaF | 0.75 ± 0.07 | 0.20 ± 0.02 | C | −2.5 | LPS heptosyltransferase II |
| rfaG | 0.7 ± 0.2 | 0.19 ± 0.04 | C | −2.6 | Glucosyltransferase I involved in LPS core biosynthesis |
| nlpD | 1.0 ± 0.1 | 0.17 ± 0.02 | OM | −2.9 | Protein related to cell division |
| tolC | 0.6 ± 0.2 | 2.0 ± 0.4 | OM | 4.0 | Porin component of several multi-drug efflux systems |
| ybgF | 0.86 ± 0.03 | 0.18 ± 0.02 | P | −2.8 | Part of the Tol-Pal contributing to maintain cell envelope integrity |
| yfgA | 0.7 ± 0.1 | 0.18 ± 0.04 | IM | −2.8 | Protein responsible for maintaining the rod shape of the E. coli cell |
| Function unknown | |||||
| nlpI | 0.7 ± 0.2 | 2.1 ± 0.1 | OM | 4.2 | Lipoprotein related to osmotic sensitivity, filamentation, and virulence |
| yfeC | 0.93 ± 0.03 | 1.4 ± 0.3 | C | 2.8 | Predicted DNA-binding transcriptional regulator |
| yieL | 0.70 ± 0.02 | 0.15 ± 0.01 | P | −3.3 | Predicted xylanase |
| yhbP | 0.63 ± 0.03 | 0.18 ± 0.01 | C | −2.8 | Function unknown. |
| yjiP | 1.10 ± 0.07 | 0.2 ± 0.1 | C | −2.6 | Predicted transposase involved in biofilm formation |
C, cytoplasm; IM, inner membrane; P, periplasm; OM, outer membrane
Fifteen mutants indentified in the initial screen which had the biggest impact on eDNA within various functional groups were further verified via qPCR with eDNA samples purified from planktonic cells cultured in LB: rna, hns, pnp, groL, cyaA, aspC, moaE, menD, pstA, rfaD, rfaG, ybgF, nlpI, yfeC, and yieL. Of these 15, the nlpI, yfeC, and rna mutants increased eDNA, and the rfaD mutant decreased eDNA as expected based on the initial screen (Fig. 1A). There was no amplification via qPCR for the eDNA samples of the hns mutant (even after a 50-fold concentration); hence, the hns deletion abolishes the formation of eDNA.
Fig. 1. eDNA quantified by qPCR.
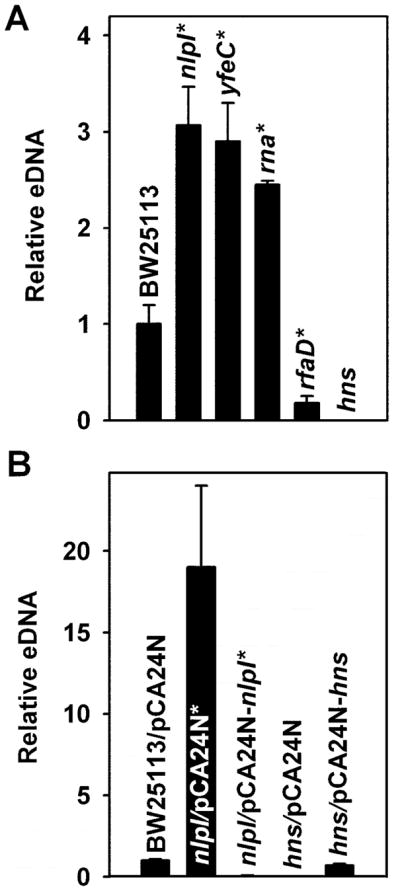
The values are the average of at least 2 independent cultures assayed in duplicate, the error bars correspond to the standard deviation, and an asterisk indicates P-values < 0.05 using Student’s T test. (A) Knock-outs mutants that altered eDNA production. Cells were grown in LB for 24 h, 250 rpm at 37°C. (B) Complementation of hns and nlpI eDNA. Cells were grown in LB for 24 h 250 rpm at 37°C with 21.5 h of induction with 0.1 mM IPTG.
Hha is a global regulator [30] with nonspecific DNA binding [31] that alters the production of multiple proteins [32] and which forms a complex with H-NS that binds DNA [31]. To evaluate if the H-NS regulation of eDNA occurs through its interaction with Hha, we evaluated via qPCR the eDNA produced by the hha mutant and hha hns double mutant. The hha mutant produces the same amount of eDNA as wild-type BW25113, and the eDNA of the hha hns mutant was not detected; therefore, H-NS regulation of eDNA is not related to Hha.
The aspC, ybgF, moaE, menD, pstA, cyaA, pnp and yieL mutants did not have statistically significant differences in their eDNA compared to the wild-type strain as assayed by qPCR. The rfaG (2.4 fold) and groL (2.9 fold) mutations increased eDNA via qPCR but decreased eDNA based on the initial screening with Quant-iT PicoGreen. These discrepancies may be due to differences in the growth conditions since the initial screen was performed with cells grown quiescently in AB minimal media supplemented with glucose and casamino acids in microtiter plates, but the cells for the qPCR screen were grown in LB media in flasks with shaking.
Complementation of nlpI and hns eDNA
The mutants with the highest impact on eDNA were nlpI and hns. To confirm that NlpI and H-NS regulate eDNA production, plasmids pCA24N-hns and pCA24N-nlpI were used to overexpress hns and nlpI (Fig. 1B). As expected, eDNA was not produced by BW25113 hns/pCA24N, and overexpressing hns in BW25113 hns/pCA24N-hns restored eDNA to 70% of the wild-type BW25113/pCA24N. Similarly, as expected, the nlpI mutation in BW25113 nlpI/pCA24N increased eDNA 19 fold while overexpressing nlpI in BW25113 nlpI/pCA24N-nlpI decreased eDNA 16 fold (Fig. 1B). Hence, our results indicate that H-NS enhances eDNA production and that NlpI negatively controls eDNA in E. coli.
Cell lysis assay
Since β-galactosidase is a cytoplasmic enzyme, its activity in culture supernatants has been used previously to determine if eDNA production occurs via lysis of a subpopulation of the culture [2,11,15,18]. Therefore, plasmid pLP170 harboring lacZ was electroporated into BW25113 containing the hns or nlpI mutations to evaluate the β-galactosidase in the culture supernatants normalized by the β-galactosidase activity of cell lysates; lacZ is inactivated in wild-type BW25113. The nlpI mutant increased cell lysis 6.4 ± 0.9 fold which is similar to its increase in eDNA (3.1 ± 0.4 fold). However, cell lysis does not explain the decrease in eDNA in the hns mutant since the deletion of hns abolished E. coli eDNA production but cell lysis decreased by 1.8 ± 0.6 fold. These results suggest that cell lysis contributes to eDNA release in E. coli; however, another mechanism may also be present.
Membrane vesicles
To investigate whether MVs were altered by the nlpI and hns mutations, we purified MVs from supernatants of BW25113 and the nlpI and hns mutants cultures made in LB medium. The nlpI mutant has 107 fold more vesicles (cf., 3-fold more eDNA) and the hns mutant has 3 fold more vesicles (cf., no eDNA) than the wild-type BW25113. These results for MVs agree with the values reported previously for the nlpI [33] and hns [34] mutants. Therefore, eDNA production via MVs is not the main mechanism of eDNA production in E. coli since the changes in MVs do not match the changes in eDNA for these two mutants.
DISCUSSION
Our results show that E. coli releases eDNA during static growth, where there are planktonic and sessile cells. Furthermore, we identified 35 proteins with a greater than 2.5-fold difference in eDNA production and characterized the nlpI and hns mutations more fully. Mutations in yfeC, rna, and nlpI increased eDNA. YfeC is an uncharacterized protein that has a helix-turn-helix domain [35]; hence, it probably is a negative transcriptional regulator of genes encoding proteins related to eDNA. rna encodes RNase I; hence, the increase in eDNA by the rna deletion may be related to the reduced degradation of DNA that occurs in the rna mutant [36]. Since RNase I is a periplasmic protein, the increase in eDNA by the rna deletion suggests that DNA is present in the periplasm which agrees with eDNA release via secretion.
The largest increase in eDNA was obtained with the nlpI mutant (Fig. 1A). NlpI is an outer membrane lipoprotein that probably participates in cell division [37] and is related to bacterial virulence in pathogenic E. coli strains by promoting adhesion to intestinal epithelial cells [38] and human brain microvascular endothelial cells [39]. The nlpI mutant shows elongation at 42°C at low osmolarity [37] and produces more than 100 fold more membrane vesicles [33]. Cells overexpressing nlpI have a prolate ellipsoidal shape and have some cells joined by partial constrictions which suggests that cell division is altered due to defects in chain elongation and the formation of the septal ring [37]. Hence, deletion of nlpI probably leads to more eDNA that may decorate the exterior of the cell and render it less able to bind epithelial and endothelial cells.
Mutations in hns and rfaD decreased eDNA. RfaD is an enzyme that participates in the synthesis of a precursor of LPS. The rfaD mutant forms mini-cells which indicate cell division defects, has a mucoid phenotype, has resistance to λ phage, and cannot growth at temperatures higher than 42°C or in media containing bile salts [40].
For the hns mutant, eDNA production was abolished since eDNA was not detected by qPCR. H-NS is an abundant protein (approximately 20,000 copies per cell) [41] that binds to the DNA condensing the nucleoid [42]. H-NS functions as a transcriptional global regulator controlling genes encoding proteins related to the cell envelope and adaptation to environmental conditions [43] including 69% of temperature regulated genes [44]. The hns mutant forms 3 fold more membrane vesicles [34] and has altered chromosome partitioning and replication [45]. Since the reduction in cell lysis by the H-NS mutants is not comparable to the reduction in eDNA production, E. coli should have another mechanism other than lysis for eDNA production. Similarly, the production of eDNA via membrane vesicles may not be the main mechanism of eDNA production in E. coli since the hns deletion increases vesiculation but decreases eDNA. Hence, it is possible that E. coli produces eDNA via direct secretion from living cells. Therefore, although speculative, our data suggest that H-NS regulates eDNA secretion in E. coli in a manner that is not dependent on Hha.
Given that E. coli is a Gram negative bacterium, to be secreted, DNA should go through the inner membrane, the cell wall, and the outer membrane. This transport of DNA may also occur through the points where the inner and outer membranes are joined to each other through the cell wall [46]. The inner and outer membrane are involved in DNA replication, and the outer membrane fractions contains newly replicated DNA. During cell division, on each side of the septum, two rings are formed where the inner and outer membranes are fussed. Since both nlpI and hns mutants have altered cell division and have the biggest effect on eDNA release, secretion in E. coli may be related to DNA replication and cell division. An eDNA secretion mechanism related to DNA replication occurs in the Gram positive Bacillus subtilis. During spore germination, B. subtillis releases eDNA following replication, and the rate of DNA synthesis is similar to the rate of DNA release [47].
RESEARCH HIGHLIGHTS.
The complete E. coli genome was screened to identify proteins that affect extracellular DNA (eDNA).
Global regulator H-NS is required for eDNA with E. coli.
Lipoprotein NlpI negatively influences eDNA production with E. coli.
The mechanism of eDNA production in E. coli may be secretion.
Acknowledgments
This research was supported by the National Science Foundation (CBET-0753702). We are grateful for the KEIO and ASKA strains provided by the Genome Analysis Project in Japan.
Abbreviations
- eDNA
extracellular DNA
- MVs
membrane vesicles
- gDNA
genomic DNA
- CSP
competence-stimulating peptide
- AHLs
acylhomoserine lactones
- dsDNA
double stranded DNA
- LB
Luria-Bertani
- qPCR
quantitative polymerase chain reaction
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 2.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci USA. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas VC, Hiromasa Y, Harms N, Thurlow L, Tomich J, Hancock LE. A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis. Mol Microbiol. 2009;72:1022–1036. doi: 10.1111/j.1365-2958.2009.06703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilain S, Pretorius JM, Theron J, Brözel VS. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl Environ Microbiol. 2009;75:2861–2868. doi: 10.1128/AEM.01317-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmsen M, Lappann M, Knochel S, Molin S. Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl Environ Microbiol. 2010;76:2271–2279. doi: 10.1128/AEM.02361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 7.Lappann M, Claus H, van Alen T, Harmsen M, Elias J, Molin S, Vogel U. A dual role of extracellular DNA during biofilm formation of Neisseria meningitidis. Mol Microbiol. 2010;75:1355–1371. doi: 10.1111/j.1365-2958.2010.07054.x. [DOI] [PubMed] [Google Scholar]
- 8.Heijstra BD, Pichler FB, Liang Q, Blaza RG, Turner SJ. Extracellular DNA and Type IV pili mediate surface attachment by Acidovorax temperans. Antonie van Leeuwenhoek. 2009;95:343–349. doi: 10.1007/s10482-009-9320-0. [DOI] [PubMed] [Google Scholar]
- 9.Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–2092. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 10.Böckelmann U, Janke A, Kuhn R, Neu TR, Wecke J, Lawrence JR, Szewzyk U. Bacterial extracellular DNA forming a defined network-like structure. FEMS Microbiol Lett. 2006;262:31–38. doi: 10.1111/j.1574-6968.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 11.Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol. 2006;59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 12.Finkel SE, Kolter R. DNA as a nutrient: novel role for bacterial competence gene homologs. J Bacteriol. 2001;183:6288–6293. doi: 10.1128/JB.183.21.6288-6293.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palchevskiy V, Finkel SE. Escherichia coli competence gene homologs are essential for competitive fitness and the use of DNA as a nutrient. J Bacteriol. 2006;188:3902–3910. doi: 10.1128/JB.01974-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinmoen H, Knutsen E, Håvarstein LS. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc Natl Acad Sci USA. 2002;99:7681–7686. doi: 10.1073/pnas.112464599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Daimon M, Awano T, Umekage S, Tanaka T, Kikuchi Y. Characterization of extracellular DNA production and flocculation of the marine photosynthetic bacterium Rhodovulum sulfidophilum. Appl Microbiol Biotechnol. 2009;84:349–356. doi: 10.1007/s00253-009-2031-7. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura S, Tanaka T, Fujita K, Itaya M, Hirashi A, Kikuchi Y. Extracellular DNA and RNA produced by a marine photosynthetic bacterium Rhodovulum sulfidophilum. Nucleic Acis Res Suppl. 2003;3:279–280. doi: 10.1093/nass/3.1.279. [DOI] [PubMed] [Google Scholar]
- 18.Palmen R, Hellingwerf KJ. Acinetobacter calcoaceticus; liberates chromosomal DNA during induction of competence by cell lysis. Current Microbiology. 1995;30:7–10. doi: 10.1007/BF00294516. [DOI] [PubMed] [Google Scholar]
- 19.Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton HL, Domínguez NM, Schwartz KJ, Hackett KT, Dillard JP. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 21.Ueda A, Wood TK. Tyrosine phosphatase TpbA of Pseudomonas aeruginosa controls extracellular DNA via cyclic diguanylic acid concentrations. Environ Microbiol Rep. 2010;2:449–455. doi: 10.1111/j.1758-2229.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- 22.Ueda A, Wood TK. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm Formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885) PLoS Pathog. 2009;5:e1000483. doi: 10.1371/journal.ppat.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Xi C. Evaluation of different methods for extracting extracellular DNA from the biofilm matrix. Appl Environ Microbiol. 2009;75:5390–5395. doi: 10.1128/AEM.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (A Complete Set of E. coli K-12 ORF Archive): Unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 27.Clark DJ, Maaløe O. DNA replication and the division cycle in Escherichia coli. J Mol Biol. 1967;23:99–112. [Google Scholar]
- 28.Ma Q, Wood TK. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol. 2009;11:2735–2746. doi: 10.1111/j.1462-2920.2009.02000.x. [DOI] [PubMed] [Google Scholar]
- 29.Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 30.García-Contreras R, Zhang XS, Kim Y, Wood TK. Protein translation and cell death: The role of rare tRNAs in biofilm formation and in activating dormant phage killer genes. PLoS ONE. 2008;3:e2394. doi: 10.1371/journal.pone.0002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieto JM, Madrid C, Prenafeta A, Miquelay E, Balsalobre C, Carrascal M, Juárez A. Expression of the hemolysin operon in Escherichia coli; is modulated by a nucleoid-protein complex that includes the proteins Hha and H-NS. Mol Gen Genet. 2000;263:349–358. doi: 10.1007/s004380051178. [DOI] [PubMed] [Google Scholar]
- 32.Balsalobre C, Johansson J, Uhlin BE, Juárez A, Muñoa FJ. Alterations in protein expression caused by the hha mutation in Escherichia coli: Influence of growth medium osmolarity. J Bacteriol. 1999;181:3018–3024. doi: 10.1128/jb.181.10.3018-3024.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006;188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem. 2002;277:32538–32545. doi: 10.1074/jbc.M203740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer ELL, Bateman A. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright M. Mutants of Escherichia coli lacking endonuclease I, ribonuclease I, or ribonuclease II. J Bacteriol. 1971;107:87–94. doi: 10.1128/jb.107.1.87-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohara M, Wu HC, Sankaran K, Rick PD. Identification and characterization of a new lipoprotein, NlpI, in Escherichia coli K-12. J Bacteriol. 1999;181:4318–4325. doi: 10.1128/jb.181.14.4318-4325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnich N, Bringer MA, Claret L, Darfeuille-Michaud A. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn’s disease. Infect Immun. 2004;72:2484–2493. doi: 10.1128/IAI.72.5.2484-2493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng CH, Tseng YT, Maruvada R, Pearce D, Xie Y, Paul-Satyaseela M, Kim KS. NlpI contributes to Escherichia coli K1 strain RS218 interaction with human brain microvascular endothelial cells. Infect Immun. 2010;78:3090–3096. doi: 10.1128/IAI.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karow M, Raina S, Georgopoulos C, Fayet O. Complex phenotypes of null mutations in the htr genes, whose products are essential for Escherichia coli growth at elevated temperatures. Res Microbiol. 1991;142:289–294. doi: 10.1016/0923-2508(91)90043-a. [DOI] [PubMed] [Google Scholar]
- 41.Dorman CJ. H-NS, the genome sentinel. Nat Rev Microbiol. 2007;5:157–161. doi: 10.1038/nrmicro1598. [DOI] [PubMed] [Google Scholar]
- 42.Dame RT, Wyman C, Goosen N. H-NS mediated compaction of DNA visualised by atomic force microscopy. Nucleic Acids Res. 2000;28:3504–3510. doi: 10.1093/nar/28.18.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hommais F, Krin E, Laurent-Winter C, Soutourina O, Malpertuy A, Le Caer JP, Danchin A, Bertin P. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol Microbiol. 2001;40:20–36. doi: 10.1046/j.1365-2958.2001.02358.x. [DOI] [PubMed] [Google Scholar]
- 44.White-Ziegler CA, Davis TR. Genome-wide Identification of H-NS-controlled, temperature-regulated genes in Escherichia coli K-12. J Bacteriol. 2009;191:1106–1110. doi: 10.1128/JB.00599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaidow A, Wachi M, Nakamura J, Magae J, Nagai K. Anucleate cell production by Escherichia coli Δhns mutant lacking a histone-like protein, H-NS. J Bacteriol. 1995;177:3589–3592. doi: 10.1128/jb.177.12.3589-3592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funnell BE. Participation of the bacterial membrane in DNA replication and chromosome partition. Trends Cell Biol. 1993;3:20–25. doi: 10.1016/0962-8924(93)90196-8. [DOI] [PubMed] [Google Scholar]
- 47.Borenstein S, Ephrati-Elizur E. Spontaneous release of DNA in sequential genetic order by Bacillus subtilis. J Mol Biol. 1969;45:137–152. doi: 10.1016/0022-2836(69)90216-2. [DOI] [PubMed] [Google Scholar]
- 48.Hong SH, Wang X, Wood TK. Controlling biofilm formation, prophage excision and cell death by rewiring global regulator H-NS of Escherichia coli. Microb Biotechnol. 2010;3:344–356. doi: 10.1111/j.1751-7915.2010.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, Goldberg JB, Pier GB. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun. 1997;65:3086–3090. doi: 10.1128/iai.65.8.3086-3090.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Misra RV, Horler RSP, Reindl W, Goryanin II, Thomas GH. EchoBASE: an integrated post-genomic database for Escherichia coli. Nucleic Acids Res. 2005;33:D329–D333. doi: 10.1093/nar/gki028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keseler IM, Collado-Vides J, Gama-Castro S, Ingraham J, Paley S, Paulsen IT, Peralta-Gil M, Karp PD. EcoCyc: a comprehensive database resource for Escherichia coli. Nucleic Acids Res. 2005;33:D334–D337. doi: 10.1093/nar/gki108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudd KE. EcoGene: a genome sequence database for Escherichia coli K-12. Nucleic Acids Res. 2000;28:60–64. doi: 10.1093/nar/28.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


