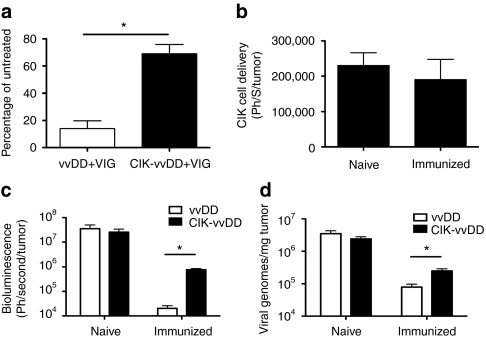
Figure 2.
CIK cells can deliver vvDD to the tumor in the face of neutralizing antibody. (a) Mice-(C57BL/6) bearing subcutaneous CMT64 tumors were pretreated with intraperitoneal (i.p.) injections of VIG (200 mg/mouse) or PBS and 12 hours later intravenous (i.v.) with vvDD-luc (1 × 107 PFU) or 1 × 107 CIK cells premixed with 1 × 107 PFU vvDD-luc for 5 hours. Viral gene expression from within the tumor was determined after 72 hours by bioluminescence imaging. Percentage of BLI signal for VIG treated relative to PBS-treated mice are shown. Mice were bled at the time of imaging to verify continued presence of neutralizing antibody in serum at this point (n = 4/group). (b) vvDD-infected CIK cells can traffic to the tumor in immunized mice. Mice either immunized with i.p. injection of WR, or naive, were implanted subcutaneously with CMT64 cells 14 days later. Once tumors had formed, they were treated i.v. with CIK-vvDD as before. CIK cells were expanded from a C57BL/6-luciferase transgenic mouse, such that they expressed luciferase. BLI (indicative of numbers of CIK cells) within the tumor were determined after 72-hours (n = 5/group). (c) Viral replication in the tumor is reduced in immunized mice. Experiment as in b repeated, only vvDD-luc and unlabeled CIK cells used, and compared to vvDD-luc delivered alone. Viral gene expression (BLI signal) within the tumor for immunized and naive mice is shown (n = 4/group) at 72 hours after treatment. (d) Experiment as in c repeated, only mice sacrificed at 72 hours after treatment and relative viral genomes in the tumor determined by Q-PCR (n = 3/group) (*P < 0.05). BLI, bioluminescence imaging; CIK, cytokine-induced killer; PBS, phosphate-buffered saline; PFU, plaque-forming units; Q-PCR, quantitative PCR; VIG, vaccinia immunoglobulin; vvDD, vaccinia virus; WR, western reserve.