Abstract
MRP RNA is a noncoding RNA component of RNase mitochondrial RNA processing (MRP), a multi-protein eukaryotic endoribonuclease reported to function in multiple cellular processes, including ribosomal RNA processing, mitochondrial DNA replication, and cell cycle regulation. A recent study predicted a potential Drosophila ortholog of MRP RNA (CR33682) by computer-based genome analysis. We have confirmed the expression of this gene and characterized the phenotype associated with this locus. Flies with mutations that specifically affect MRP RNA show defects in growth and development that begin in the early larval period and end in larval death during the second instar stage. We present several lines of evidence demonstrating a role for Drosophila MRP RNA in rRNA processing. The nuclear fraction of Drosophila MRP RNA localizes to the nucleolus. Further, a mutant strain shows defects in rRNA processing that include a defect in 5.8S rRNA processing, typical of MRP RNA mutants in other species, as well as defects in early stages of rRNA processing.
Keywords: Drosophila, RNAse MRP, ribosomal RNA processing, noncoding RNA
INTRODUCTION
MRP RNA was initially identified as a component of RNase mitochondrial RNA processing (MRP), an essential, highly conserved eukaryotic ribonucleoprotein complex that also contains approximately 10 proteins (Yuan et al. 1991; Schmitt and Clayton 1992; Chamberlain et al. 1998; Welting et al. 2004; Salinas et al. 2005). Studies in Saccharomyces cerevisiae and mammals have identified several RNase MRP substrates, associated with diverse cellular processes that are cleaved in a site-specific manner. In mammalian cells, RNase MRP was implicated in processing mitochondrial transcripts complementary to the origin of replication of the mitochondrial chromosome, to produce primers for mitochondrial DNA replication (Chang and Clayton 1987). In S. cerevisiae, it was known for some time to cleave the rRNA precursor at a single site, leading to the formation of the 5′ end of 5.8S rRNA (Schmitt and Clayton 1993; Lygerou et al. 1996). However, the results of a recent study show that inactivation of MRP RNA results in additional rRNA processing defects that occur in early stages of the pathway (Lindahl et al. 2009).
Cleavage of B-type cyclin (CLB2) mRNA mediated by RNase MRP to regulate cell cycle progression was also observed in S. cerevisiae (Gill et al. 2004). In human cells, a defect in rRNA processing and regulation of cyclin B mRNA were also observed; however, direct cleavage of these substrates by RNase MRP has not yet been demonstrated (Hermanns et al. 2005; Thiel et al. 2005, 2007). Recently, human MRP RNA (RMRP) was identified as a component of a novel complex with telomerase reverse transcriptase (TERT) that functions as an RNA-dependent RNA polymerase. In this complex, RMRP acts as a template for the synthesis of dsRMRP RNA, primed by a 3′ fold-back structure. dsRMRP RNA associates with Ago2 and is processed into 22-nucleotide (nt) dsRNAs in a Dicer-dependent fashion. It has been proposed that these dsRNAs represent a novel class of endogenous siRNAs that regulate RMRP levels by a negative feedback mechanism (Maida et al. 2009).
MRP RNA was the first noncoding RNA to be associated with a human disease. Ridanpää et al. (2001) described a set of mutations in the RMRP gene that cosegregate with the phenotype of the autosomal recessive disease cartilage-hair hypoplasia (CHH). Various RMRP mutations are now known to be associated with other disorders such as Omenn syndrome and anauxetic dysplasia (Thiel et al. 2005; Martin and Li 2007). Short stature is common to all of these diseases; however, each disease presents a characteristic spectrum of other defects affecting a broad range of organs (Makitie et al. 2001a,b; Martin and Li 2007; Toiviainen-Salo et al. 2008). Recent studies have identified correlations between specific molecular defects and disease symptoms in patients with various RMRP mutations. Mutations that affect rRNA processing are correlated with the characteristic anauxetic dysplasia symptom of severe skeletal defects. Mutations that result in increased cyclin B mRNA levels are correlated with symptoms characteristic of CHH. These patients show less severe skeletal defects but have higher incidences of immunodeficiency, hematological abnormalities, and cancer (Thiel et al. 2005, 2007).
We report the expression of the Drosophila ortholog of MRP RNA (CR33682), previously predicted by a bioinformatics screen for MRP RNA sequences (Fig 1A; Piccinelli et al. 2005). Characterization of a mutant strain shows that Drosophila MRP (dMRP) is an essential gene. dMRP mutants display a severe impairment in growth, a characteristic shared with human diseases carrying mutations in this gene (Martin and Li 2007). These phenotypic defects could be the result of impairments at different stages of rRNA processing that we have observed. These include the classic defect in processing 5.8S rRNA that has been associated with human and S. cerevisiae RNase MRP mutants (Schmitt and Clayton 1993; Lygerou et al. 1996; Hermanns et al. 2005; Thiel et al. 2005), as well as a defect in early rRNA processing similar to a defect recently reported in S. cerevisiae (Lindahl et al. 2009).
FIGURE 1.
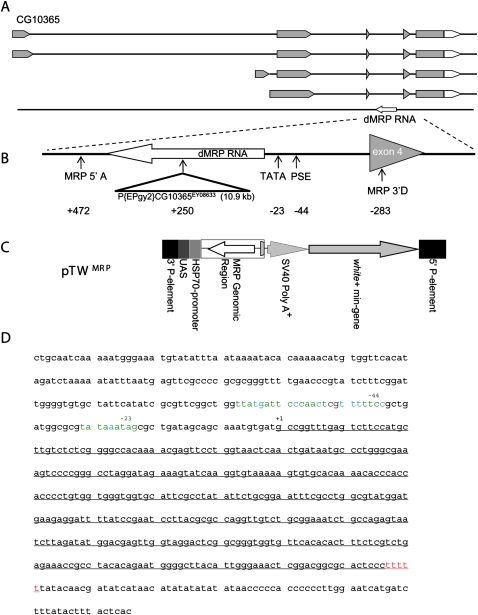
The Drosophila (dMRP) RNA gene. (A) Piccinelli et al. (2005) predicted a potential dMRP RNA encoded by a gene located in the third intron of CG10365, transcribed from the opposite strand. (Gray) Translated sequences. (B) Expanded view of the dMRP gene. P{EPgy2} CG10365EY08633 disrupts dMRP in the dMRPEY08633 mutant strain. Positions of primers used to amplify the genomic region used to make the pTW -MRP strain (MRP 5′A and MRP 5′D) are indicated. (C) Structure of pTW MRP used to make the pTW -MRP strain. Regions inserted into chromosome 2 by untargeted integration via 5′ and 3′ P-element sequences are shown. dMRP genomic sequences were inserted in the opposite orientation relative to the UAS/Hsp70 transcriptional regulatory sequences so that dMRP RNA was expressed from its own regulatory sequences. The white (+) mini-gene encodes a red eye-color gene for selecting transgenic individuals. (D) dMRP sequence with potential Pol III regulatory elements. The sequence of the entire intron containing dMRP is shown. Transcribed sequences are underlined. Positions of PSE (−44 nt) and TATA-like element (−23 nt) upstream of the transcription start site (+1) are indicated above the sequence. (Green nucleotides in promoter elements) Identical nucleotides in all reference sequences (Hernandez et al. 2007), (blue nucleotides) the same as at least one of the reference sequences, (red nucleotides) consensus Pol III terminator (Hernandez et al. 2007).
RESULTS
dMRP RNA is expressed throughout the Drosophila life cycle and localizes to the nucleolus
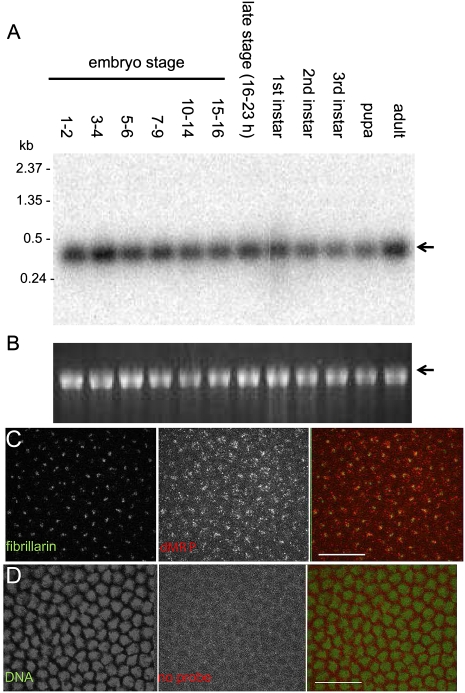
Expression of dMRP RNA was detected throughout the Drosophila life cycle (Fig. 2A,B). This is consistent with its role as a component of RNase MRP in the essential cellular processes of ribosome biogenesis, mitochondrial DNA replication, and cell cycle regulation. The major fraction of RNase MRP in S. cerevisiae (Schmitt and Clayton 1993) and MRP RNA in mammalian cells is in the nucleolus, specifically in the dense fibrillar component marked by fibrillarin (Li et al. 1994; Jacobson et al. 1995). In syncytial Drosophila embryos, dMRP RNA colocalizes with Drosophila fibrillarin in the nucleolus (Fig. 2C). Similar results were observed in S2 cells (data not shown). Nucleolar localization of dMRP RNA suggests that this is a conserved feature of MRP RNA.
FIGURE 2.
Expression and nucleolar localization of dMRP RNA. (A) dMRP RNA is expressed throughout Drosophila development. Northern blot of total Drosophila RNA probed with dMRP antisense RNA. dMRP RNA (arrow) is predicted to be 383 nt long, including the termination signal. (B) rRNA (arrow) as a loading control is visualized by ethidium bromide staining. (C) dMRP RNA colocalizes with fibrillarin in syncytial Drosophila embryos. dMRP RNA was detected by FISH, fibrillarin with rabbit anti-Drosophila fibrillarin antibody. (D) Control FISH without RNA probe. DNA stained with PicoGreen was used as a counterstain instead of fibrillarin to show a lack of nuclear localization of low signal levels in the red channel. Fibrillarin distribution varies considerably with cell cycle and does not consistently mark each nucleus. (C,D) Images of a single confocal plane. Scale bar, 200 μm.
Characterization of a dMRP mutant strain
Rescue of a dMRP mutant strain with genomic DNA containing an intact dMRP gene
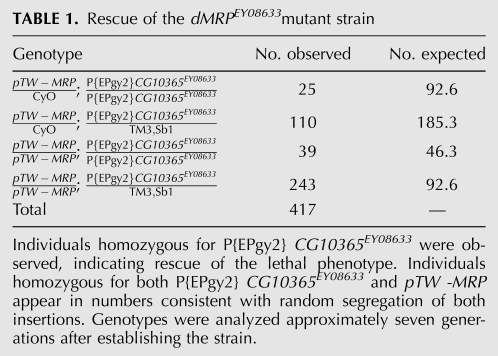
dMRP is encoded within an intron of the uncharacterized gene CG10365, but is transcribed in the opposite direction. We determined that a P-element, P{EPgy2}CG10365EY08633, inserted in transcribed dMRP sequences results in a lethal phenotype (Fig. 1A). Due to the large size of the P-element (10.9 kb), it is unlikely that a product expressed from the dMRP RNA polymerase III (Pol III) promoter could form a product with dMRP RNA function. This product would likely be degraded, resulting in a functional null mutation. We note that this P-element is inserted in an intron of the surrounding CG10365 gene, and as such does not disrupt its translated sequences. Thus, it is possible that the lethal phenotype was caused by disruption of the dMRP gene, leaving CG10365 function largely intact. To test this idea, a P-element vector (pTWMRP) containing a genomic fragment that encompassed the entire intronic region of the dMRP gene was inserted into chromosome II and used to rescue the lethal P-element insertion. The pTWMRP transgene was then tested in P{Epgy2} CG10365EY08633 mutants (Table 1). Individuals homozygous for the dMRP mutation P{Epgy2} CG10365EY08633 and the pTWMRP transgene were present in numbers consistent with random segregation of both insertions, suggesting a full rescue of the lethal phenotype in these individuals. The viability of dMRP mutant individuals carrying pTWMRP indicates that the mutant phenotype resulting from the insertion of P{Epgy2} CG10365EY08633 is caused by an impairment of the dMRP gene and not CG10365. CG10365 may therefore be a nonessential gene, or the P{Epgy2} CG10365EY08633 insertion may not impair its function to a physiologically significant degree. As the phenotypes associated with P{Epgy2} CG10365EY08633 appear to be specific to impairment of dMRP function, we will hereafter refer to this mutation as dMRPEY08633.
TABLE 1.
Rescue of the dMRPEY08633mutant strain
dMRPEY08633 mutants are impaired in growth and development and die as second instar larvae
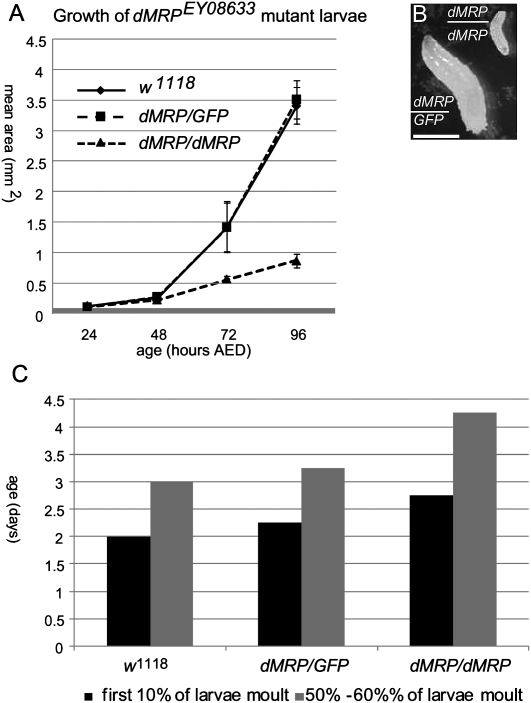
A significant defect in growth of homozygous dMRPEY08633 mutants compared with the reference w1118 strain was observed beginning at 3 d after egg deposition (AED) (Fig. 3A). At 5 d AED the mean cross-sectional area of homozygous dMRPEY08633 mutants was approximately one-third that of the w1118 strain. Homozygous dMRPEY08633 mutants were also delayed in development, undergoing the first molt ∼1 d later than normal. Despite the defects in growth and delayed development, homozygous dMRPEY08633 mutants showed no gross physical deformities. Growth and development of heterozygous dMRPEY08633 larvae were indistinguishable from the normal w1118 strain (Fig. 3A,B).
FIGURE 3.
Homozygous dMRPEY08633 mutant larvae are delayed in growth and development. (A) dMRPEY08633/dMRPEY08633 mutants showed a significant growth delay (mean cross sectional area for 20 individuals) beginning before 72 h AED compared with control (w1118) or heterozygous dMRPEY08633/GFP siblings. (B) Comparison of 5-d-old dMRPEY08633/dMRPEY08633 and dMREY08633/GFP mutant individuals. Scale bar, 1 mm. (C) The first molt in dMRPEY08633/dMREY08633 mutants occurred ∼1 d later than control (w1118) or heterozygous dMRPEY08633/GFP siblings raised in the same vial(s). (dMRP) dMRPEY08633.
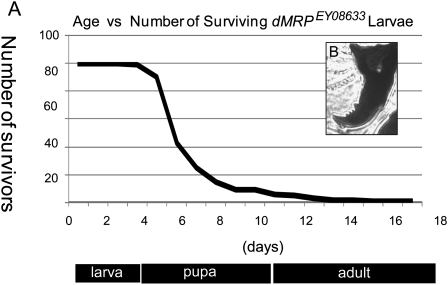
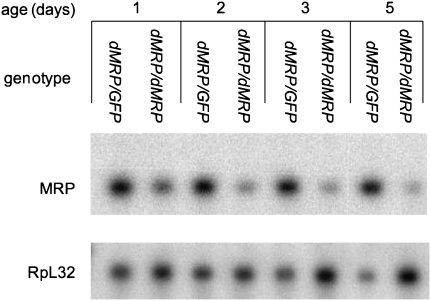
Homozygous dMRPEY08633 mutants showed a bimodal pattern of mortality, with ∼70% of individuals dying between 5 and 6 days of age while the remaining individuals lingered as second instar larvae for up to 17 d AED (Fig. 4A), which is several days after normal individuals have reached adulthood. Mouth hooks examined after death had two to four teeth, characteristic of the second instar stage (Fig. 4B; Apatov 1929). Northern blot analysis of dMRP RNA expression levels showed a progressive decline in homozygous dMRPEY08633 mutants from 1 to 5 d of age while levels remained constant in dMRPEY08633/GFP heterozygotes (Fig. 5). This progressive decline in dMRP RNA levels is likely due to gradual depletion of maternal dMRP RNA and correlates with the timing of impairments in growth and development prior to mortality.
FIGURE 4.
Homozygous dMRPEY08633 mutants die during the second larval instar. (A) A bimodal pattern of mortality was observed, with ∼70% of individuals dying 5–6 d AED. The remaining individuals lingered as second instar larvae for up to 17 d compared with control populations, which reached adulthood at ∼10 d AED. (B) Representative mouth hook from a homozygous dMRPEY08633 larva examined post mortem. All mouth hooks had two to four teeth, indicative of the second instar stage. (Below) Approximate development timeline for wild-type individuals. (dMRP) dMRPEY08633.
FIGURE 5.
dMRP RNA levels decline at the time of growth arrest and death in dMRPEY08633 mutants. A Northern blot of total RNA from homozygous dMRPEY08633/dMRPEY08633 mutants and their heterozygous dMRPEY08633/GPF siblings during larval development shows levels of dMRP RNA (MRP). The same blot was probed for RpL32 mRNA (RpL32) as a loading control. (dMRP) dMRPEY08633.
dMRPEY08633 mutants show impaired rRNA processing
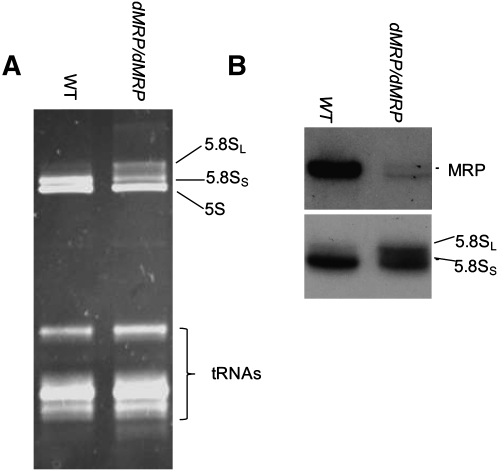
To identify a potential physiological defect affecting growth and/or development of dMRPEY08633 mutants, we examined the well-documented role for MRP RNA as a component of RNase MRP in the endonucleolytic cleavage of the rRNA precursor leading to formation of the 5′ end of 5.8S rRNA. Mutations in S. cerevisiae and human MRP RNAs result in accumulation of unprocessed or long forms of 5.8S RNA (5.8SL) relative to the processed short form (5.8SS) (Schmitt and Clayton 1993; Lygerou et al. 1996; Hermanns et al. 2005; Thiel et al. 2005). Our analysis of the relative abundance of these two forms of 5.8S rRNA in dMRPEY08633 mutant larvae showed an increase in the abundance of the 5.8SL form (Fig. 6). This result is consistent with a defect in a subset of previously described RNase MRP mutants that has been described in other species (Schmitt and Clayton 1993; Lygerou et al. 1996; Hermanns et al. 2005; Thiel et al. 2005, 2007).
FIGURE 6.
5.8S rRNA processing is impaired in dMRP mutants. Total RNA isolated from wild-type (WT) and dMRPEY08633 mutant larvae was separated in a denaturing polyacrylamide gel and either directly analyzed by staining with ethidium bromide (A) or used for Northern blotting with probes specific to the dMRP RNA or 5.8 rRNA (B). The two forms of 5.8S rRNA are indicated. (dMRP) dMRPEY08633, (WT) Oregon R strain.
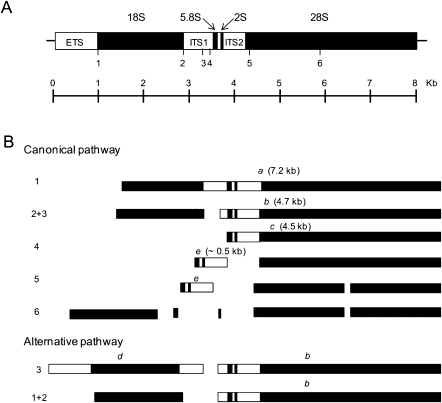
A recent study identified an additional role for S. cerevisiae RNase MRP in early stages of rRNA processing. In this study, a reduction in levels of normal early intermediates was observed in MRP RNA mutants, which was accompanied by the appearance of abnormal intermediates (Lindahl et al. 2009). To investigate a potential role for dMRP in regulating early processing steps, we examined rRNA intermediates in homozygous dMRPEY08633 mutants. Figure 7 is a schematic representation of two pathways for processing the primary pre-rRNA transcript in Drosophila melanogaster. The canonical pathway is analogous to that described in vertebrates (Levis and Penman 1978). An alternative pathway was subsequently proposed by Long and Dawid (1980) that differs from the canonical pathway in the order of early cleavages steps.
FIGURE 7.
A D. melanogaster ribosome gene and previously described rRNA processing pathways. (A) The primary rRNA transcript is drawn approximately to scale. (Solid boxes) Regions that are processed into mature rRNA. ETS (external transcribed spacer), ITS1 (internal transcribed spacer 1), and ITS2 (internal transcribed spacer 2) (open boxes) are removed during processing. Endonucleolytic cleavage sites are indicated by numbered marks (1–6). (B) The canonical and alternative rRNA processing pathways that have been previously described in D. melanogaster. Processing intermediates designated according to Long and Dawid (1980) are indicated by letters above each fragment.
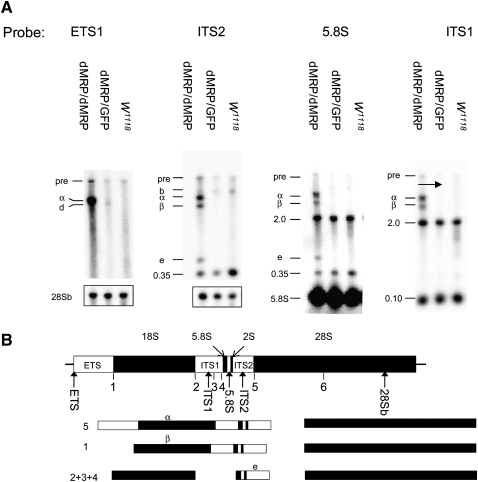
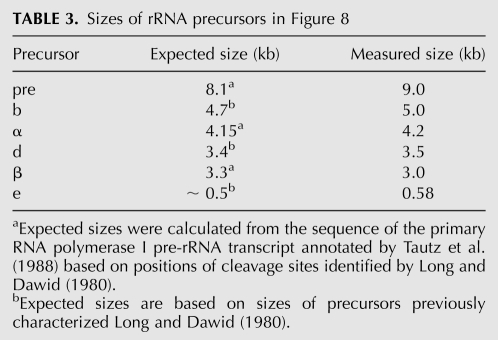
Northern blots of total RNA from homozygous dMRPEY08633 mutants, dMRPEY08633/GFP heterozygotes of the same age, and w1118 second instar larvae were hybridized with oligonucleotide probes (oligos) complementary to several regions of the primary pre-rRNA transcript (Fig. 8B; Table 2). RNA levels for each genotype were normalized for similar levels of mature rRNA to compare processed with unprocessed rRNA levels. Probing with an oligo complementary to the external transcribed spacer (ETS) revealed a 4.2-kb RNA species in homozygous dMRPEY08633 mutants that was not detected in phenotypically normal larvae. A 4.2-kb molecule (designated intermediate α) was also seen when blots were probed with oligos complementary to the internal transcribed spacer (ITS)1, ITS2, and 5.8S regions (Fig. 8A). This molecule spans from the 5′ end of the primary transcript to the ITS2 region. Assuming that it is a product of a cleavage at one of the normal sites, it would be the product of an initial cleavage at site 5 (Fig. 7). A 3.0-kb molecule (designated intermediate β) was detected only in RNA from homozygous dMRPEY08633 mutants on blots probed with ITS1, ITS2, and 5.8S oligos but not the ETS oligo (Fig. 8A). This could represent the product of intermediate α, following cleavage at site 1, which removes the ETS from the primary transcript. The experimentally determined sizes of both of these molecules are consistent with their expected sizes, which were determined by comparison with sizes of previously characterized precursors (Long and Dawid 1980) or calculated from the sequence of the primary RNA polymerase I pre-rRNA transcript based on the annotation by Tautz et al. (1988) (see Table 3). Neither of these molecules corresponds to products of either of the previously described Drosophila rRNA processing pathways (Fig. 7).
FIGURE 8.
rRNA precursors in dMRPEY08633 mutants and a novel processing pathway that they may represent. (A) Northern blot analysis of rRNA precursors in dMRPEY08633/dMRPEY08633 mutants (5 d AED), their heterozygous dMRPEY08633/GPF siblings (5 d AED), and second instar wild-type (WT) (w1118) larvae. Loading was normalized to similar levels of mature rRNA. Blots probed with ETS1 and ITS2 oligos were subsequently probed with a 28Sb oligo to show similar loading. Similar loading for the blot probed with the 5.8S oligo is indicated by the mature 5.8S product. The blot probed with ITS2 was stripped and reprobed with ITS1. The primary transcript (pre) and mature products are indicated. Novel processing intermediates are identified by letters that correspond to intermediates shown in the pathway below. Previously characterized intermediates b (seen in RNA from dMRPEY08633/GPF larvae probed with ETS probe) and d (seen in RNA from dMRPEY08633/GPF and w1118 larvae) probed with ITS2 probes are identified as in Figure 7. Fragments of unknown identities are indicated by their apparent molecular weight (kb). 5.8S and ITS1 oligos both hybridized to a 2.0-kb fragment that is approximately the size of the 28Sb mature rRNA and could result from nonspecific hybridization to this abundant RNA species. The 0.35-kb and 0.10-kb fragments seen with the 5.88 and ITS1 probes, respectively, do not correspond to known products of the Drosophila rRNA processing pathway. They may represent novel intermediates of may result from nonspecific hybridization with unrelated small RNAs. (B) Map of the primary rRNA transcript is represented as in Figure 7. (Arrows) Positions of oligonucleotide hybridization probes. Probes are complementary to the following regions: ETS, ITS2, 5.8S, ITS1, and 28S. Initial cleavages of the rRNA processing pathway represented by these fragments are shown below the primary transcript. Further processing is likely to follow the canonical pathway.
TABLE 2.
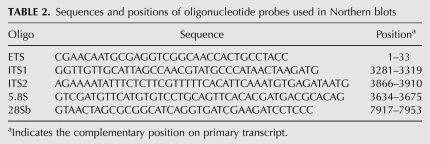
Sequences and positions of oligonucleotide probes used in Northern blots
TABLE 3.
Sizes of rRNA precursors in Figure 8
Accumulation of a 0.58-kb molecule (designated intermediate e) was detected in RNA from homozygous dMRPEY08633 mutants with ITS2 and 5.8S oligos but not with ETS or ITS1 oligos (Fig. 8). The size (Table 3) and structure of this molecule suggest that it may correspond to a previously observed normal precursor of 5.8S and 2S rRNAs, designated intermediate e by Long and Dawid (1980). However, the boundaries of both of these intermediates need to be defined more accurately to determine their relationship. Intermediate e, identified in Figure 8, could be the product of further processing of intermediate β or result from abnormal accumulation of an intermediate produced by a normal processing pathway. We were unable to detect this intermediate in RNA from phenotypically normal larvae. If intermediate is a processed product of intermediate β, then the three intermediates α, β, and that accumulates in homozygous dMRPEY08633 mutants may represent a series of intermediates in an atypical processing pathway generated by cleavages at normal sites used in an atypical order (Fig. 8B).
Our probes detected several RNAs that were present in RNA from homozygous dMRPEY08633 mutants as well as the two phenotypically normal genotypes, whose identities we could not determine. 5.8S and ITS1 oligos both hybridized to a 2.0-kb fragment that is close in size to the 28Sb mature rRNA and could result from nonspecific hybridization to this abundant RNA species. The 0.35-kb fragment seen with the ITS2 and 5.8S probes and the 0.10-kb fragment seen with the ITS1 probe do not correspond to known products of Drosophila rRNA processing pathways. They may represent novel processing intermediates or may be detected as a result of nonspecific hybridization with unrelated small RNAs.
DISCUSSION
Drosophila MRP RNA is orthologous to MRP RNA genes in other species
Our results support the idea that dMRP RNA shares structural and functional homology with conserved MRP RNA genes previously characterized in other eukaryotes. In addition to the structural and sequence similarities between dMRP RNA and MRP RNAs in other species described by Piccinelli et al. (2005), we identified genomic sequences flanking dMRP RNA resembling RNA Pol III regulatory elements (Fig. 1; Hernandez et al. 2007). The presence of these elements suggests that in Drosophila, as in most species, MRP RNA is transcribed by RNA Pol III (Dieci et al. 2007). Our success in rescuing the dMRPEY08633 mutant strain with a genomic fragment containing these sequences is consistent with the idea that these are functional dMRP transcriptional regulatory sequences.
A number of additional observations support the idea that this gene is orthologous to the gene encoding MRP RNA in other eukaryotes. Essentially uniform expression of dMRP RNA throughout the Drosophila life cycle (Fig. 2A) is consistent with its role in fundamental cellular processes such as ribosome biogenesis, mitochondrial DNA replication, and cell cycle regulation (Chang and Clayton 1987; Schmitt and Clayton 1993; Lygerou et al. 1996; Gill et al. 2004; Thiel et al. 2007). Further, dMRP RNA localizes to the nucleolus, the same compartment to which the bulk of S. cerevisiae RNase MRP (Gill et al. 2006) and mammalian MRP RNA localize (Fig. 2C; Li et al. 1994; Jacobson et al. 1995).
The rRNA processing pathway has not been characterized as extensively in Drosophila as in other species. There have been few additional studies of this pathway since mapping of cleavage sites by Long and Dawid (1980). In their study, a single form of 5.8S was identified. We have identified both long and short forms of 5.8S rRNA in normal larvae (Fig. 6). Our data also reveal similarities in rRNA processing between dMRP RNA and MRP RNA orthologs in other species. S. cerevisiae and human MRP RNA genes have been known for some time to cleave the rRNA precursor leading to the formation of the 5′ end of the 5.8SS rRNA. A reduction in the level of 5.8SS, accompanied by an increase in the level of the unprocessed 5.8SL, in MRP RNA mutants is a strong indication of this function (Schmitt and Clayton 1993; Lygerou et al. 1996; Hermanns et al. 2005; Thiel et al. 2005, 2007). Homozygous dMRPEY08633mutants display a similar change in relative abundances of the two forms of 5.8S rRNAs, indicating a similar function for this gene in Drosophila (Fig. 6).
Drosophila and S. cerevisiae MRP RNA genes show similar effects on early rRNA processing
We have observed an additional defect in early rRNA processing events that resembles one that was recently reported in S. cerevisiae MRP RNA mutants. In these mutants, an atypical 24S intermediate, spanning from the 5′ end of the ETS to the 3′ end of 5.8S, was detected. It was proposed that this intermediate was generated by a premature cleavage in ITS2 followed by 3′ to 5′ exonuclease trimming to the 3′ end of 5.8S (Lindahl et al. 2009). The 24S intermediate resembles intermediate α in homozygous dMRPEY08633 mutants (Fig. 8). Intermediate α, like the 24S intermediate, begins with the ETS at the 5′ end. ITS2 sequences at the 3′ end suggest that it was generated by premature cleavage at the normal cleavage site 5 (Fig. 7). However, unlike the 24S intermediate, intermediate α retains ITS2-derived sequences (Fig. 8). Accumulation of intermediate β and intermediate e in addition to intermediate α suggests further processing of intermediate α. Together, these fragments could represent a series of intermediates in an atypical processing pathway (Fig. 8).
Normally, the pre-rRNA transcript is cleaved at defined sites in a consistent order to produce a defined set of rRNA intermediates that are ultimately processed into mature rRNAs. To account for the atypical pathway in homozygous dMRPEY08633 mutants, we propose a role for RNAse MRP in maintaining the order of early cleavage steps. Specifically, RNase MRP could contribute to suppressing premature cleavage at site 5 in ITS2 (Fig. 7). This function could be performed as part of a large complex, associating with the pre-rRNA and masking cleavage site 5. Subsequent conformational changes in this complex could expose site 5 for cleavage at the appropriate processing step. At a later stage in the pathway RNase MRP would perform its previously documented function of an endoribonuclease, cleaving the pre-rRNA precursor at a single site in ITS1 to process the 5′ end of the 5.8S rRNA.
Similar aberrant intermediates in Drosophila and S. cerevisiae mutant MRP RNA genes could be formed by a common mechanism, such as the one described above. Alternatively, they could form as a result of the mechanism proposed by Lindahl et al. (2009). In this mechanism RNase MRP could function as an endoribonuclease to directly cleave the pre-rRNA transcript at multiple sites. In addition to its previously characterized role of processing the 5′ end of the 5.8S rRNA, it would also cleave early intermediates. The absence of this activity in MRP RNA mutants this would lead to reduction of normal early rRNA precursors. The 24S intermediate would be produced in the absence of this activity while cleavage in ITS2 continues.
Elevated levels of atypical intermediates in the pathway outlined in Figure 8 could accumulate as a result of inefficient processing. Normally, early cleavages of the primary pre-rRNA transcript separate the 18S region from the 28S region, followed by further processing along separate pathways to form distinct 40S and 60S ribosomal subunits (Fatica and Tollervey 2002). In intermediate α of the atypical pathway, the 5.8S and 2S regions are inappropriately linked to the 18S region, which is destined to become part of the 40S subunit. An inability by the normal pathway to process intermediate α could result in an increase in its levels. Inefficient processing of intermediate α by an atypical pathway could lead to accumulation of additional intermediates such as intermediates β and e. Eventually, accumulation of atypical intermediates surpassing critical levels could interfere with normal rRNA processing pathways and lead to defects in ribosome biogenesis. This model is consistent with a reduction in levels of normal early processing intermediates in S. cerevisiae MRP RNA mutants reported by Lindahl et al. (2009) and predicts that analysis of physical and morphological features of ribosomal subunits or nucleoli where they are assembled could reveal additional defects.
Intermediate e resembles a precursor of 5.8S and 2S rRNAs previously identified by Long and Dawid (1980). Both molecules span from 5.8S to ITS2; however, their precise boundaries have not yet been determined. Based on analogy with the S. cerevisiae rRNA processing pathway (Fatica and Tollervey 2002), two forms of intermediate e would be expected in normal individuals, one the product of processing initiated by RNase MRP cleavage and another unprocessed form. In Drosophila, intermediate b is the likely substrate for these alternative cleavages (Fig. 7). At this time we cannot determine which of the two predicted forms of this intermediate (or perhaps both) we detect in homozygous dMRPEY08633 mutants since their size difference is likely to be very small. In S. cerevisiae there is only a 7-nt difference between the two forms (Shuai and Warner 1991). However, accumulation of intermediate e in homozygous dMRPEY08633 mutants with reduced levels of dMRP RNA suggests that it could be the precursor of the long form of the 5.8S rRNA that has not been cleaved by RNase MRP. Determining the precise 5′ boundary of this intermediate would determine if it is indeed the 5.8SL precursor. If intermediate e in homozygous dMRPEY08633 mutants is a precursor of 5.8 SL, it is tempting to speculate that the pathway in Fig. 8B could function in normal individuals as a result of incomplete suppression of premature cleavage in ITS2 to produce low levels of 5.8 SL.
Mutation of dMRP phenotypically resembles human RMRP mutants
The similarity in phenotype of the dMRPEY08633 mutant strain with human patients with RMRP mutations further supports the idea of functional homology between these genes. The impairment in growth of dMRPEY08633 mutant larvae resembles the short stature thought to be caused by an intrinsic defect in proliferation of cells in human patients with RMRP mutations (Pierce and Polmar 1982). It is not clear which of the three RNase MRP functions may be causing the defects in growth and development seen in the dMRPEY08633 mutants. It is unlikely that it is an impairment of cell cycle regulation by a defect in cyclin B mRNA degradation because cyclins A, B, and B3 are not expressed in endoreplicating cells (Lehner and O'Farrell 1989, 1990), which constitute most of the larval tissues. The dMRPEY08633 mutant phenotype may be most similar to a class of human RMRP mutations that is associated with impaired rRNA processing that does not alter cyclin B mRNA degradation. This class of mutations is associated with a severe growth defect seen in the disease anauxetic dysplasia.
The potential impact of a defect in mitochondrial function on dMRPEY08633 mutants cannot be inferred from studies of human RMRP mutations because defects in mitochondrial function have not yet been detected in these patients (Hermanns et al. 2005). Indeed a potential role for RNase MRP in mitochondrial DNA replication has been controversial. The enzyme was initially isolated from mouse cells and shown to cleave an RNA representing the primer for mitochondrial DNA replication (Chang and Clayton 1987). This activity was dependent on complementarity of a segment of MRP RNA to sequences of the RNA substrate (Bennett and Clayton 1990). However, the cleavage site on the substrate is 6–10 nt from the in vivo cleavage site, suggesting that the in vitro activity may be an artifact (Kiss and Filipowicz 1992). In favor of a role for RNase MRP in mitochondria, there is good evidence for localization of MRP RNA to mitochondria of mouse cardiomyocytes by in situ hybridization (Li et al. 1994). The recent purification of a mitochondrial enzyme consisting of MRP RNA with a protein composition distinct from nucleolar RNase MRP provides convincing evidence for a mitochondrial function in S. cerevisiae (Lu et al. 2010).
In Drosophila, mutations in a variety of mitochondrial functions have been described. These mutations can affect a number of diverse processes such as apoptosis (Abdelwahid et al. 2007), spermatogenesis (Hales and Fuller 1997), and growth in both mitotic and endoreplicating tissues (Morris et al. 2008). The latter effect is consistent with the impairment in growth displayed by dMRPEY08633 mutants. We have been unable to detect localization of dMRP RNA to mitochondria in several tissues that we examined, with the exception of occasional localization to mitochondria in third instar larval muscle (data not shown). Additional analysis employing a variety of experimental approaches could establish whether dMRP RNA functions in mitochondria. These could include additional attempts at localization of dMRP RNA to mitochondria by in situ hybridization as well as purification of RNase MRP activity from mitochondria. In Drosophila and other metazoans, identifying a mitochondrial RNase MRP would be more likely in tissues that are known to be actively replicating mitochondrial DNA.
The defects that we report in growth of dMRPEY08633 mutants are consistent with impaired rRNA processing and/or impaired mitochondrial functions, based on phenotypic comparisons of other genetic defects in these processes. Defects in development may be an indirect consequence of growth defects. In Drosophila larvae there may be a requirement to reach a certain size before proceeding to the next stage of development.
We have shown that the single Drosophila homolog of MRP functions analogously to mammalian MRP. The well-characterized RNAi pathway in Drosophila (Kim et al. 2009) together with the recent discovery of RMRP-derived endogenous siRNAs in human cells (Maida et al. 2009) facilitates future studies of the relatively unexplored area of RNAi-mediated regulation of noncoding RNAs. Our identification of a Drosophila strain with a mutation in MRP RNA will be a useful tool in these future endeavors.
MATERIALS AND METHODS
Drosophila culture and strains
Drosophila melanogaster strains were maintained at 18°C or 25°C according to standard maintenance procedures on semisolid medium consisting of 1.2% brewer's yeast, 1.2% agar, 8% cornmeal, 7.5% v/v blackstrap molasses supplemented with 0.25 g of methyl 4-hydroxybenzoate (Sigma, dissolved in 1.5 mL of 95% ethanol) as a preservative. The following stocks, obtained from the Bloomington Drosophila Stock Center, were used: the normal w1118 strain; second chromosome balancer CyO/Sco, third chromosome balancers w-; TM3 Sb1/Ly and w-; Sb1/TM3 P(w+mC = ActGFP) JMR2,Ser1 and strains bearing the lethal P-element insertion in CG10365; P[EPgy2] CG10365EY08633.
Generation of transgenic animals
A genomic region that included the dMRP gene was PCR-amplified from pBAC11E7 (GenBank accession no. AC008201.8) with MRP5′A: CACCCGTTGAGGACAAAGAGGTGAGTA and MRP3′D: GCTGCTTGAGATAATCCAGTGCCG primers that were derived from flanking CG10365 coding sequences and cloned into pENTR/D (Invitrogen) to make pENTRMRP. This entry vector was then recombined into pTW (provided by T. Murphy, Carnegie Institute, Troy, MI), inserting the dMRP gene into pTW to make pTWMRP. Note that the dMRP genomic region was inserted in the opposite orientation to the UAS promoter in this vector so that dMRP RNA would be expressed from its own regulatory sequences. Wild-type w1118 flies were transformed with pTWMRP to make the w-; pTW -MRP strain.
Observations of Drosophila growth and development
Embryos were collected on apple juice-agar plates. After hatching, larvae were maintained at 25°C on the same plates supplemented with yeast paste spread for the period of observation. Homozygous dMRPEY08633 mutants were identified as non-GFP individuals of the strain P{Epgy2} CG10365EY08633/w+; Sb1/TM3 P (w+mC = ActGFP) JMR2,Ser1 (abbreviated as dMRP/GFP). The lethal stage of mutant larvae was determined by observation of mouth hooks mounted under #1.5 coverslips in standard tissue mounting medium (5 g of Mowiol 40-88, 20 mL of PBS, 10 mL of glycerol, 2.5% DABCO [1,4-diazabicyclo(2.2.2)octane]).
rRNA processing analysis
Total RNA isolated from Oregon-R and dMRPEY08633mutant larvae (∼2.5 μg per lane) was separated in an 8%/7 M urea polyacrylamide denaturing gel and either stained with ethidium bromide for direct RNA visualization or transferred to Hybond-N+ membrane (Amersham Biosciences) for Northern analysis with 32P-labeled probes specific to the MRP RNA or 5.8S rRNA (see below). Templates for probe labeling were generated by PCR using the primers MRP fwd: 5′-AAGTCCCCGGGCCTAGGATAGAAAG-3′; MRP rev: 5′-CGGTTTCTCAGACGAGAAAGTGTGTG-3′; 5.8S rRNA fwd: 5′-AACTCTAGGCGGTGGATCACTCGGC-3′; 5.8S rRNA rev: 5′-CAGCATGGACTGCGATATGCGTTCA-3′. Note: 5.8S primers were chosen from the sequence annotated as CR40454 (Flybase). For analysis of early rRNA intermediates, oligonucleotide probes were 32P end-labeled with [γ-32P]ATP (3000 Ci/mmol, 10 mCi/mL, Perkin Elmer) using T4 polynucleotide kinase (Invitrogen) by the forward reaction according to the manufacturer's protocol.
RNA extraction and Northern blot analysis
Total RNA was extracted using TRIzol (Invitrogen) from adults and staged collections throughout development. Equal amounts of denatured RNA (1–2 μg) were loaded in RNA gel loading buffer (Eppendorf); 2.5–5 μL of buffer for each 1.0 μL of RNA. Redistilled formamide (Invitrogen) was added to samples to a minimum of 60% final concentration to allow fractionation on a native 2% agarose gel (Masek et al. 2005). RNA was capillary-transferred to a Brightstar-Plus Membrane (Ambion) and UV cross-linked with a 120-mJ burst for 30 sec. The membrane was pre-hybridized in 5–10 mL of hybridization buffer (3 M urea, 5× SSC, 0.1% [w/v] N-laurylsarcosine, 0.02% SDS, 0.5% BSA, 0.1 mg/mL sonicated salmon sperm DNA) in a glass hybridization tube for 1 h at 60°C.
Hybridization buffer was replaced with 5 mL of fresh buffer containing the appropriate antisense RNA probe labeled with [α-32P]UTP, and the membrane was hybridized overnight at 60°C. A probe encompassing the entire transcribed region of dMRP (Piccinelli et al. 2005) was used to detect dMRP RNA. This region was PCR-amplified with MRP forward (GCCGGTTTGAGTCTTCC) and MRP reverse (TAATACGACTCACTATAGGGAAAAAAAGTGCGCCG) primers from pBAC11E7. The antisense probe was transcribed with T7 polymerase from T7 promoter sequences added to the template in the MRP reverse primer. The membrane was stripped and hybridized to a probe detect the RpL32 gene as a loading control. The template for RpL32 (CG7939) was amplified from the cDNA RH03940 using T3 and T7 primers from the corresponding promoter sequences in the polylinker of pFLC-1. The antisense probe was transcribed using T3 polymerase. Probes were labeled with 32P in a 20-μL transcription reaction containing T7/T3 Buffer (Invitrogen); 0.5 mM each of ATP, CTP, and GTP; 12 μM UTP; 20 U of SUPERase-In (Ambion); 50 U of T7 polymerase (Invitrogen); 100 ng of DNA template; and 50 μCi of [α-32P]UTP (800 Ci/mmol, 20 mCi/mL, Perkin Elmer).
In situ hybridization
Transcribed dMRP sequences were PCR-amplified from pBAC11E7 with the primers MRP fwd GCCGGTTTGAGTCTTCC and MRP rev GGAGTGCGCCGTCCGAGTT. Using this product as a template, a subregion of the dMRP gene, consisting of nucleotides 126–366 with T7 promoter sequences appended, was amplified in a subsequent step using the primers MRP2 5′ CACAAAACACCCACCCCTGTG and MRP-2 3′ + T7 TAATACGACTCACTATAGGGTCCGAGTTTCCCAATGTAA. A digoxygenin-labeled antisense RNA probe transcribed from this template was hybridized to fixed embryos as described in Hughes and Krause (1999). RNA probes were visualized by fluorescent antibody staining using a primary sheep anti-DIG antibody (1:200, Roche) and secondary anti-sheep Alexi-conjugated fluorescent antibody (1:2000, Molecular Probes). Fibrillarin was detected with rabbit anti-fibrillarin antibody (1:4000, Abcam).
ACKNOWLEDGMENTS
We thank Julie Haskins, Andrew Symes, Keri Wilson, and Fatima Pirani for their technical help with this project and members of the Simmonds and Matera laboratories for helpful discussion and support. This work was supported by grant 84154 from the Canadian Institutes of Health Research to A.J.S. and grant R01-GM053034 from the National Institutes of Health to A.G.M. M.D.S. was supported by a studentship from the Alberta Heritage Foundation for Medical Research. A.K.B. was supported by an undergraduate student research award from the National Sciences and Engineering Research Council of Canada. A.J.S. is an Alberta Innovates–Health Solutions Senior Scholar.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2227710.
REFERENCES
- Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K 2007. Mitochondrial disruption in Drosophila apoptosis. Dev Cell 12: 793–806 [DOI] [PubMed] [Google Scholar]
- Apatov WW 1929. Growth and variation of the larvae of Drosophila melanogaster. J Exp Zool 52: 407–437 [Google Scholar]
- Bennett JL, Clayton DA 1990. Efficient site-specific cleavage by RNase MRP requires interaction with two evolutionarily conserved mitochondrial RNA sequences. Mol Cell Biol 10: 2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JR, Lee Y, Lane WS, Engelke DR 1998. Purification and characterization of the nuclear RNase P holoenzyme complex reveals extensive subunit overlap with RNase MRP. Genes Dev 12: 1678–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DD, Clayton DA 1987. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J 6: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A 2007. The expanding RNA polymerase III transcriptome. Trends Genet 23: 614–622 [DOI] [PubMed] [Google Scholar]
- Fatica A, Tollervey D 2002. Making ribosomes. Curr Opin Cell Biol 14: 313–318 [DOI] [PubMed] [Google Scholar]
- Gill T, Cai T, Aulds J, Wierzbicki S, Schmitt ME 2004. RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: Novel method of mRNA degradation. Mol Cell Biol 24: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T, Aulds J, Schmitt ME 2006. A specialized processing body that is temporally and asymmetrically regulated during the cell cycle in Saccharomyces cerevisiae. J Cell Biol 173: 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales KG, Fuller MT 1997. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 90: 121–129 [DOI] [PubMed] [Google Scholar]
- Hermanns P, Bertuch AA, Bertin TK, Dawson B, Schmitt ME, Shaw C, Zabel B, Lee B 2005. Consequences of mutations in the non-coding RMRP RNA in cartilage-hair hypoplasia. Hum Mol Genet 14: 3723–3740 [DOI] [PubMed] [Google Scholar]
- Hernandez G Jr, Valafar F, Stumph WE 2007. Insect small nuclear RNA gene promoters evolve rapidly yet retain conserved features involved in determining promoter activity and RNA polymerase specificity. Nucleic Acids Res 35: 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SC, Krause HM 1999. Single and double FISH protocols for Drosophila. Methods Mol Biol 122: 93–101 [DOI] [PubMed] [Google Scholar]
- Jacobson MR, Cao LG, Wang YL, Pederson T 1995. Dynamic localization of RNase MRP RNA in the nucleolus observed by fluorescent RNA cytochemistry in living cells. J Cell Biol 131: 1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC 2009. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139 [DOI] [PubMed] [Google Scholar]
- Kiss T, Filipowicz W 1992. Evidence against a mitochondrial location of the 7-2/MRP RNA in mammalian cells. Cell 70: 11–16 [DOI] [PubMed] [Google Scholar]
- Lehner CF, O'Farrell PH 1989. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell 56: 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, O'Farrell PH 1990. The roles of Drosophila cyclins A and B in mitotic control. Cell 61: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R, Penman S 1978. Processing steps and methylation in the formation of the ribosomal RNA of cultured Drosophila cells. J Mol Biol 121: 219–238 [DOI] [PubMed] [Google Scholar]
- Li K, Smagula CS, Parsons WJ, Richardson JA, Gonzalez M, Hagler HK, Williams RS 1994. Subcellular partitioning of MRP RNA assessed by ultrastructural and biochemical analysis. J Cell Biol 124: 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L, Bommankanti A, Li X, Hayden L, Jones A, Khan M, Oni T, Zengel JM 2009. RNase MRP is required for entry of 35S precursor rRNA into the canonical processing pathway. RNA 15: 1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO, Dawid IB 1980. Alternative pathways in the processing of ribosomal RNA precursor in Drosophila melanogaster. J Mol Biol 138: 873–878 [DOI] [PubMed] [Google Scholar]
- Lu Q, Wierzbicki S, Krasilnikov AS, Schmitt ME 2010. Comparison of mitochondrial and nucleolar RNase MRP reveals identical RNA components with distinct enzymatic activities and protein components. RNA 16: 529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lygerou Z, Allmang C, Tollervey D, Seraphin B 1996. Accurate processing of a eukaryotic precursor ribosomal RNA by ribonuclease MRP in vitro. Science 272: 268–270 [DOI] [PubMed] [Google Scholar]
- Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, Okamoto N, Kasim V, Hayashizaki Y, Hahn WC, Masutomi K 2009. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461: 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makitie O, Kaitila I, Rintala R 2001a. Hirschsprung disease associated with severe cartilage-hair hypoplasia. J Pediatr 138: 929–931 [DOI] [PubMed] [Google Scholar]
- Makitie OM, Tapanainen PJ, Dunkel L, Siimes MA 2001b. Impaired spermatogenesis: An unrecognized feature of cartilage-hair hypoplasia. Ann Med 33: 201–205 [DOI] [PubMed] [Google Scholar]
- Martin AN, Li Y 2007. RNase MRP RNA and human genetic diseases. Cell Res 17: 219–226 [DOI] [PubMed] [Google Scholar]
- Masek T, Vopalensky V, Suchomelova P, Pospisek M 2005. Denaturing RNA electrophoresis in TAE agarose gels. Anal Biochem 336: 46–50 [DOI] [PubMed] [Google Scholar]
- Morris JZ, Bergman L, Kruyer A, Gertsberg M, Guigova A, Arias R, Pogorzelska M 2008. Mutations in the Drosophila mitochondrial tRNA amidotransferase, bene/gatA, cause growth defects in mitotic and endoreplicating tissues. Genetics 178: 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli P, Rosenblad MA, Samuelsson T 2005. Identification and analysis of ribonuclease P and MRP RNA in a broad range of eukaryotes. Nucleic Acids Res 33: 4485–4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF, Polmar SH 1982. Lymphocyte dysfunction in cartilage-hair hypoplasia: Evidence for an intrinsic defect in cellular proliferation. J Immunol 129: 570–575 [PubMed] [Google Scholar]
- Ridanpää M, van Eenennaam H, Pelin K, Chadwick R, Johnson C, Yuan B, vanVenrooij W, Pruijn G, Salmela R, Rockas S, et al. 2001. Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia. Cell 104: 195–203 [DOI] [PubMed] [Google Scholar]
- Salinas K, Wierzbicki S, Zhou L, Schmitt ME 2005. Characterization and purification of Saccharomyces cerevisiae RNase MRP reveals a new unique protein component. J Biol Chem 280: 11352–11360 [DOI] [PubMed] [Google Scholar]
- Schmitt ME, Clayton DA 1992. Yeast site-specific ribonucleoprotein endoribonuclease MRP contains an RNA component homologous to mammalian RNase MRP RNA and essential for cell viability. Genes Dev 6: 1975–1985 [DOI] [PubMed] [Google Scholar]
- Schmitt ME, Clayton DA 1993. Nuclear RNase MRP is required for correct processing of pre-5.8S rRNA in Saccharomyces cerevisiae. Mol Cell Biol 13: 7935–7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Warner JR 1991. A temperature sensitive mutant of Saccharomyces cerevisiae defective in pre-rRNA processing. Nucleic Acids Res 19: 5059–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D, Hancock JM, Webb DA, Tautz C, Dover GA 1988. Complete sequences of the rRNA genes of Drosophila melanogaster. Mol Biol Evol 5: 366–376 [DOI] [PubMed] [Google Scholar]
- Thiel CT, Horn D, Zabel B, Ekici AB, Salinas K, Gebhart E, Ruschendorf F, Sticht H, Spranger J, Muller D, et al. 2005. Severely incapacitating mutations in patients with extreme short stature identify RNA-processing endoribonuclease RMRP as an essential cell growth regulator. Am J Hum Genet 77: 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CT, Mortier G, Kaitila I, Reis A, Rauch A 2007. Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum. Am J Hum Genet 81: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toiviainen-Salo S, Kajosaari M, Piilonen A, Makitie O 2008. Patients with cartilage-hair hypoplasia have an increased risk for bronchiectasis. J Pediatr 152: 422–428 [DOI] [PubMed] [Google Scholar]
- Welting TJ, van Venrooij WJ, Pruijn GJ 2004. Mutual interactions between subunits of the human RNase MRP ribonucleoprotein complex. Nucleic Acids Res 32: 2138–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Tan E, Reddy R 1991. The 40-kilodalton to autoantigen associates with nucleotides 21 to 64 of human mitochondrial RNA processing/7-2 RNA in vitro. Mol Cell Biol 11: 5266–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]