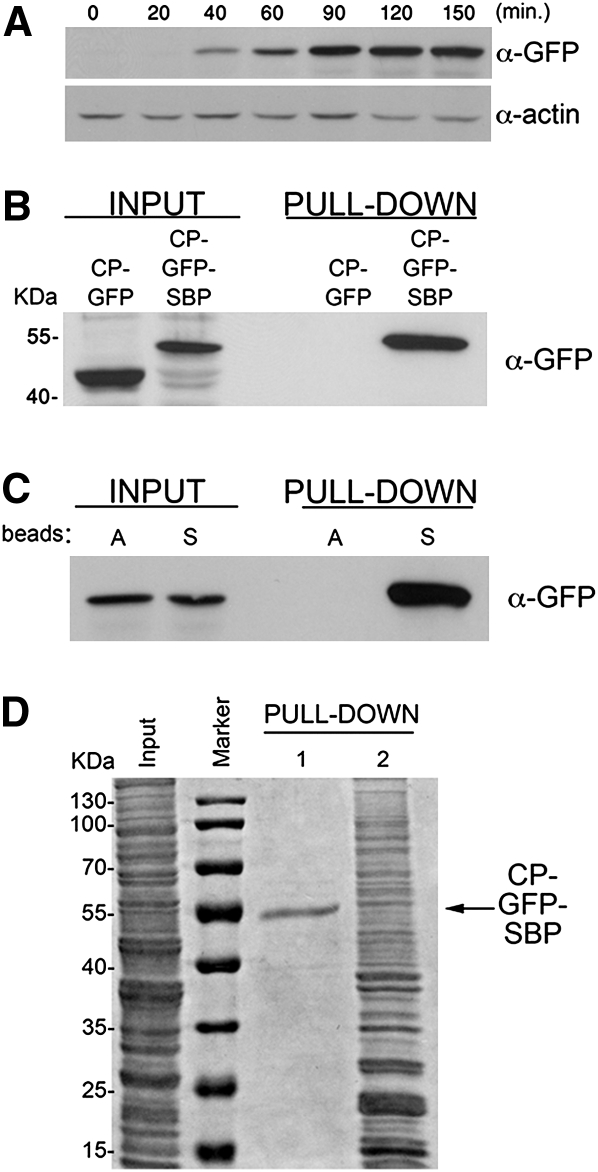
FIGURE 2.
Characterization of the MS2-CP-GFP-SBP fusion protein. (A) Starvation-induced expression of MS2-CP-GFP-SBP. Yeast transformed with pMS2-CP-GFP-SBP plasmid were grown to mid-log phase (O.D.600 ≅ 1) in synthetic medium, shifted to medium lacking methionine, grown for the indicated times (minutes), and then collected. Cells were lysed and 50 μg of protein samples were analyzed by Western blotting using anti-GFP antibodies to detect MS2-CP-GFP-SBP or anti-actin antibodies to detect actin, as a loading control. (B) MS2-CP-GFP-SBP, but not MS2-CP-GFP, is efficiently pulled down with streptavidin-conjugated beads. Yeast transformed with plasmids expressing MS2-CP-GFP or MS2-CP-GFP-SBP were grown in 200-mL cultures to mid-log phase, starved for methionine for 75 min, and harvested. Following lysis, 5 mg of total protein extract derived from each transformant was incubated with streptavidin beads, washed with lysis buffer, and eluted using free biotin. Eluates (Pull-down) were resolved by SDS-PAGE along with 50-μg samples of total protein (Input) and probed with anti-GFP antibodies. Molecular mass is given in kilodaltons (kDa). (C) MS2-CP-GFP-SBP does not bind to immobilized avidin. Yeast transformed with pMS2-CP-GFP-SBP were grown to mid-log phase in 400-mL cultures, incubated in medium lacking methionine for 75 min, and harvested. After lysis, separate aliquots of 8 mg of total protein were incubated overnight with beads conjugated to either avidin (A beads) or streptavidin (S beads). Following washing, proteins were eluted with biotin and both the eluates (Pull-down) and 50 μg of samples of total protein (Input) were analyzed by Western blotting using anti-GFP antibodies. (D) Avidin blocking and biotin-mediated elution greatly improve the signal-to-noise ratio. Yeast transformed with pMS2-CP-GFP-SBP were grown in 400-mL cultures, starved for methionine for 75 min, lysed, and two aliquots each of 2 mg of total protein were incubated overnight with streptavidin-conjugated beads. One aliquot was blocked for 1 h with free avidin prior to the pull-down, and the bound material was eluted by competition with free biotin (PULL-DOWN 1), while the second aliquot (PULL-DOWN 2) was not avidin blocked and was eluted by boiling in sample buffer containing 0.1 M dithiothreitol for 5 min. Both eluates were resolved by SDS-PAGE along with a 50-μg aliquot of total protein (Input) and were stained with a general protein stain (Imperial; Sigma).