FIGURE 6.
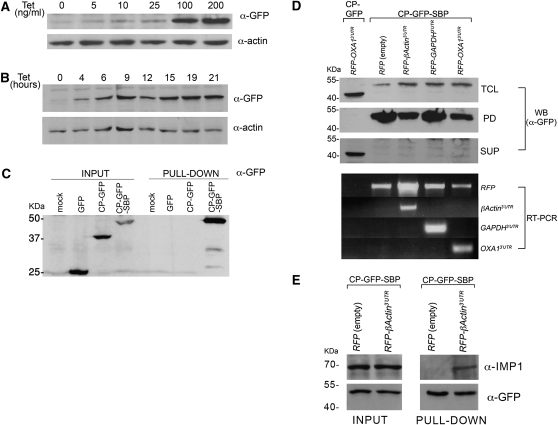
Use of RAPID in animal cells. (A) Dose-dependent induction of MS2-CP-GFP-SBP expression. 293TRex cells were transiently transfected with pcDNA4-MS2-CP-GFP-SBP (6 μg of DNA/10-cm dish), harvested after 8 h, and reseeded into 6-well plates. After an additional 12 h, tetracycline was added at the indicated concentration and the cells were grown for an additional 20 h. After harvesting, the cells were lysed and 10% of the total protein extract was resolved by SDS-PAGE and analyzed in Western blots using anti-GFP to detect MS2-CP-GFP-SBP and anti-actin antibodies to detect actin, as a loading control. (B) Time-dependent induction of MS2-CP-GFP-SBP expression. Stable 293TRex-MS2-CP-GFP-SBP cells were grown to ∼50% confluency, and then tetracycline was added to the medium (100 ng/mL) for the indicated times. After harvesting, the cells were lysed and 30 μg of the total protein extract from each time point was resolved by SDS-PAGE and analyzed in blots, as described in A. (C) Mammalian expressed MS2-CP-GFP-SBP precipitates with immobilized streptavidin. HEK293 cells were transiently transfected with pcDNA4/TO plasmids coding for GFP, MS2-CP-GFP, or MS2-CP-GFP-SBP (8 μg of DNA/100-mm dish). Nontransfected HEK293 cells were also included (mock). After 24 h, the cells were harvested, total protein was extracted, and 3.5 mg from each sample was taken for pull-down with streptavidin-conjugated beads. Both the eluates (PULL-DOWN) and 40 μL of the total protein extract (INPUT) samples were resolved by SDS-PAGE and analyzed by Western blotting using anti-GFP antibodies. (D) Isolation of mammalian MS2L-tagged mRNAs using RaPID. 293TRex cells stably expressing MS2-CP-GFP-SBP were transfected (6 μg of DNA/100-mm dish) with pN-RFPX24 plasmids expressing HA-RFP alone or as a fusion with the 3′UTRs of the β-Actin, GAPDH, or OXA1 mRNAs, as indicated. In addition, 293TRex cells stably expressing MS2-CP-GFP were transfected (6 μg of DNA/100-mm dish) with a pN-RFPX24 plasmid expressing HA-RFP fused to the 3′UTR of OXA1 mRNA. The cells were treated with tetracycline (100 ng/mL, for 12 h), collected, cross-linked with 0.1% formaldehyde, lysed, and 5 mg of total cellular extract from each sample was taken for RaPID. For Western analysis, 25% of the eluate (PD), 30 μg of total protein (TCL), and 30 μg of the supernatant remaining after incubation with the streptavidin beads (SUP) were resolved by SDS-PAGE and detected in blots using anti-GFP antibodies. For RT–PCR analysis (RT–PCR), RNA was isolated from the remaining 75% of the eluate, subjected to reverse transcription, and analyzed by PCR using the indicated primers. (E) Precipitation of endogenous IMP1 with human β-Actin mRNA. 293TRex cells stably expressing MS2-CP-GFP-SBP were transfected (10 μg of DNA/10-cm dish) with either pN-RFPX24 (empty) or pN-RFPX24 containing the 3′UTR of β-Actin. Cells were grown in the presence of tetracycline (100 ng/mL) for 12 h, collected, cross-linked using 0.01% formaldehyde, and 18 mg of each total extract was processed by RaPID. The eluates and 50 μg of the total input were analyzed by Western blots using the indicated antibodies.