Table 1.
Development of the first stereocontrolled SM coupling of chiral haloallenes.
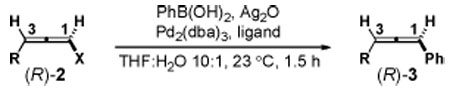 | ||||||
|---|---|---|---|---|---|---|
| Entry | 2 | R | X | ligand | 3 | % stereoretention a,b |
| 1 | (R)-2a | t-Bu | CI | PPh3 | (S)-3a | −78 |
| 2 | (R)-2b | t-Bu | Br | PPh3 | (S)-3a | −78 |
| 3 | (R)-2c | t-Bu | I | PPh3 | (R)-3a | 72 |
| 4 | (R)-2d | 3-pentyl | I | PPh3 | (R)-3b | 58 |
| 5 | (R)-2e | n-pentyl | I | PPh3 | (R)-3c | 25 |
| 6 | (R)-2c | t-Bu | I | PFur3 | (R)-3a | 80 |
| 7 | (R)-2c | t-Bu | I | PCy3 | (R)-3a | 50 |
| 8 | (R)-2c | t-Bu | I | Pt-Bu2Me | (R)-3a | 71 |
| 9 | (R)-2c | t-Bu | I | Po-Tol3 | (R)-3a | 91 |
| 10 | (R)-2c | t-Bu | I | Pt-Bu3 | (R)-3a | 93 |
| 11 | (R)-2c | t-Bu | I | XPhos | (R)-3a | 91 |
| 12c | (R)-2c | t-Bu | I | XPhos | (R)-3a | >99d |
| 13c | (R)-2d | 3-pentyl | I | XPhos | (R)-3b | >99 |
| 14c | (R)-2e | n-pentyl | I | XPhos | (R)-3c | 85 |
% stereoretention = ee product/ee starting material (chiral GC, average of 2 runs); negative values reflect net stereoinversion.
Unoptimized GC yields ranged from 10–83%.
Hexane:THF:H2O 9:1:1 was used as solvent.
Isolated yield = 61%.
