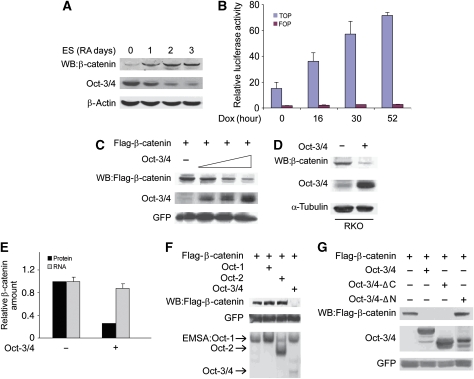
Figure 1.
Oct-3/4 promotes β-catenin degradation through its N-terminal domain. (A) Western blot (WB) analysis of Oct-3/4 and β-catenin levels during retinoic acid (RA)-induced ES cell differentiation. Cells were treated with RA for 0–3 days. β-actin served as a loading control. (B) ZHBTc4 ES cells were transfected with TOPFlash or FOPFlash expression vectors. Luciferase activity was assayed in untreated cells and cells treated with dox for the indicated time points. (C) 293T cells were transfected with Flag-β-catenin and GFP expression vectors, together with increasing amounts of Oct-3/4 expression plasmid (1, 5 and 10 μg). β-catenin and Oct-3/4 protein levels were detected using anti-Flag and anti-Oct-3/4, antibodies, respectively. GFP was used as a transfection efficiency control. (D) RKO cells were infected with lentiviral Oct-3/4 expression vector or empty control vector. WB analyses of Oct-3/4 and endogenous β-catenin protein levels were performed using antibodies directed against Oct-3/4 and β-catenin. The α-tubulin served as a loading control. (E) A relative quantification of endogenous β-catenin protein (black bars) and β-catenin mRNA (grey bars) levels in Oct-3/4 transfected (+) or untransfected (−) RKO cells. Ubiquitin C (UBC) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were used as loading controls for real-time quantitative PCR. (F) The 293T cells were transfected with Oct-1, Oct-2 and Oct-3/4 expression vectors. β-catenin degradation was shown by WB analysis. GFP served as a loading control. Cell extracts were incubated with P32-end-labelled Octa oligonucleotide, and Oct-1, Oct-2 and Oct-3/4 binding was detected using EMSA. (G) The 293T cells were transfected with the indicated expression vectors. Both Oct-3/4 and β-catenin levels were determined using WB analysis. GFP was used as a transfection efficiency control.