Abstract
Myeloid differentiation factor 88 (MyD88), an adaptor critical for innate immune function, plays a role in neutrophil recruitment and myocardial injury after transient ischemia. However, how MyD88 signaling modulates neutrophil function and myocardial injury remains unclear. In an in vivo model of neutrophil migration and a chimeric model of MyD88 deletion, we demonstrated that Gr-1-positive (Gr-1+) neutrophil migration was significantly decreased by 68% in MyD88-deficient (Myd88−/−) mice and by 33% in knockout→wild-type (KO→WT; donor→recipient) chimeric mice, which lacked MyD88 in bone marrow cells but maintained normal MyD88 expression in the heart. This marked attenuation in neutrophil migration was associated with decreased peritoneal neutrophil CXCR2 expression and lower peritoneal KC, a neutrophil chemoattractant, in MyD88−/− mice. Moreover, in vitro, KC induces significantly more downregulation of CXCR2 expression in MyD88−/− than WT neutrophils. In an in vivo model of myocardial ischemia-reperfusion (I/R) injury, KO→WT chimeric mice had significantly smaller infarct sizes compared with the WT→WT mice. While there was a marked increase in proinflammatory cytokine/chemokine expression in the myocardium following I/R, there was no significant difference between WT→WT and KO→WT mice. In contrast, Gr-1+ neutrophil recruitment in the myocardium was markedly attenuated in MyD88−/− mice. Deletion of Toll-interleukin-1 receptor (TIR)-domain-containing adaptor protein-inducing interferon-β-mediated transcription factor (Trif), another innate immune adaptor, had no effect on the KC-mediated CXCR2 downregulation or on myocardial neutrophil recruitment after I/R. Taken together, these findings suggest that MyD88 signaling is essential for maintaining neutrophil migratory function and chemokine receptor expression. MyD88 signaling in bone marrow-derived circulating cells may play a specific and critical role in the development of myocardial I/R-induced injury.
Keywords: ischemia, reperfusion, MyD88, Toll-like receptor, inflammation
neutrophils represent the first line of defense against invading pathogens and play a critical role in host response in both infectious and noninfectious tissue inflammation such as ischemia-reperfusion (I/R) injury (38). Evidence from several lines of investigation suggests that inflammation is an important functional contributor to the development of ischemic myocardial injury (15, 24, 30, 38). Following I/R, there is a robust activation of multiple soluble and cellular factors including activation of endothelial cells, release of chemoattractants, and rapid recruitment of neutrophils into ischemic myocardium, all of which contribute to subsequent myocardial injury (38). While these inflammatory responses have been well studied, the precise signaling mechanisms that control these critical events remain incompletely understood.
Innate immune systems such as those mediated via Toll-like receptors (TLRs) are important host defense mechanism against microbial infection. There are at least 10 TLRs identified so far. They recognize and specifically bind to a variety of pathogenic agonists by “molecular pattern recognition” (19). In addition to their pivotal role in host immune defense, recent studies have suggested that TLRs, such as TLR-4 and TLR-2, play a critical role in tissue inflammatory injury in response to noninfectious insults in various organs, including the heart (6, 10, 29).
TLR signaling can be divided into two general pathways, namely, Myeloid differentiation factor 88 (MyD88)- and Toll-interleukin-1 receptor (TIR)-domain-containing adaptor protein-inducing interferon-β-mediated transcription factor (Trif)-dependent (or MyD88-independent) pathways. The two distinct signaling pathways lead to the production of proinflammatory cytokines and type 1 IFN, respectively (25). All TLRs, with the exception of TLR-3, signal through a common MyD88-dependent pathway (6, 19). MyD88−/− mice (18) lack the ability to respond to lipopolysaccharide although MyD88-independent pathways (e.g., Trif-dependent) exist in TLR-4 signaling (20, 37). Trif is also critical for the TLR-3 signaling pathway (37). In mouse models of myocardial I/R injury, previous studies have established that global TLR-4- or MyD88-deficient mice have reduced myocardial neutrophil infiltration and are partially protected from myocardial I/R injury (10, 14, 29). However, the underlying mechanisms by which TLR signaling modulates neutrophil recruitment and myocardial injury remain incompletely understood. Here we tested the hypotheses 1) that MyD88 signaling controls neutrophil migratory function by modulating tissue chemoattractant production and neutrophil chemokine receptor expression and 2) that MyD88 signaling in bone marrow-derived circulating cells plays an important role in the pathogenesis of myocardial I/R injury.
METHODS
Animals.
MyD88−/− mice were generated by Kawai and colleagues (18) and had been backcrossed >10 generations into the C57BL/6J strain. Trif−/− mice were generated by Yamamoto et al. (37). The animal protocol was approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital.
MyD88 chimeric mice.
Six- to eight-week-old mice were lethally irradiated with a dose of 10 Gy and transplanted with total of 107 bone marrow cells isolated from either wild-type (WT) or MyD88−/− mice. There was a high mortality among MyD88−/− mice subjected to irradiation and bone marrow transfusion even at lower dose (6 Gy) of irradiation. Thus, all recipient mice used in the current study were WT. Eight weeks later, MyD88 expression of both bone marrow-derived and myocardial cells in the recipient mice were examined and carefully characterized. For details, see Supplemental Methods and Fig. S1; Supplemental Material for this article is available online at the Journal website.
Western blot analysis.
Homogenates of heart tissues or cell pellets were centrifuged at 12,000 g at 4°C for 30 min. Proteins were separated in 4–20% gradient SDS-PAGE and immunoblotted with MyD88 antibody (1:500 diluted) as described previously (7, 9).
Quantitative RT-PCR.
Quantitative (q)RT-PCR was performed as described previously (8, 14). Changes in relative gene expression normalized to 18S rRNA levels were determined using the relative CT method (where CT is the threshold cycle number). Oligonucleotides primers are listed in Supplemental Methods.
In vivo neutrophil migratory function.
Peritoneal cells were elicited by intraperitoneal injection of 1 ml of 4% thioglycollate. At the end of protocol, 3 ml of normal saline with 0.2% EDTA was injected into the peritoneum. Peritoneal lavage fluids were mixed thoroughly and collected. Total cell counts (except red blood cells) were manually performed. Neutrophil percentage was determined by flow cytometry using specific anti-mouse Gr-1 (anti-Ly-6G and Ly6C).
Flow cytometry.
Peritoneal or myocardial cells were incubated with anti-CD16/CD32 mAbs (BD Biosciences). Subsequently, they were incubated with phycoerythrin-conjugated anti-mouse CXCR2 mAb (R&D Systems) and APC-conjugated anti-mouse Gr-1 mAb (BD Biosciences) at 4°C for 60 min. The antibody-labeled cells were washed, fixed, and analyzed by flow cytometry. We also gated Gr-1-positive (Gr-1+) cells with anti-Ly-6G, which is more specific for neutrophils. We found that 79%, 94%, and 96% of Gr-1+ cells from the peritoneal lavage, bone marrow, and the heart, respectively, were Ly-6G+, whereas 99% of Ly-6G+ cells were also Gr-1+ (Table S1 and Fig. S3 in Supplemental Results).
Multiplex cytokine immunoassay.
Multiple cytokines were simultaneously measured in the myocardial tissues or peritoneal lavage using a Luminex multiplex bead-based immunoassay (Milliplex MAP Mouse cytokine kit) according to the manufacturer's protocol. A set of purified cytokines was included in the assay to calculate cytokine levels in the samples.
Bone marrow neutrophil isolation.
Bone marrow cells were removed from both the femur and the tibia. Cells were washed once and layered onto a Histopaque gradient (1,077–1,119) and centrifuged for 30 min at 700 g (without brake). Neutrophils were concentrated at the lower interface, and the neutrophil purity was >90% as examined by Gr-1 and Ly-6G antibodies staining and flow cytometry.
I/R injury model.
Left thoracotomy was performed and left anterior descending coronary artery (LAD) ligated with 1 silk sutures (7.0) ∼2 mm from its origin with a slip knot. Myocardial ischemia was confirmed by myocardial blanching and by ECG. For I/R injury, after 30 min, the LAD ligature was released (suture was left in place for late use) and the chest was closed. In the sham-operated animals, a slip knot tie was passed under the LAD but not tied. The overall mortality rates of the I/R injury model were <10% and were similar among all four animal groups.
Area-at-risk and myocardial infarction measurements.
Area-at-risk (AAR) and myocardial infarction (MI) were measured as described previously (14). (For details, see Supplemental Methods.)
Myocardial leukocyte isolation.
The hearts were digested and myocardial leukocytes were isolated and analyzed using flow cytometry. To determine whether enzyme digestion had any effect on neutrophil measurement, neutrophils were isolated from bone marrow by density gradient and subjected to digestion buffer used for myocardial cell preparation. The cells were then stained and gated with Gr-1 and Ly-6G antibodies in flow cytometry. For details, see Supplemental Results and Fig. S4.
Statistical analysis.
Unless stated otherwise, all data are expressed as means ± SD and were analyzed with one-way or two-way ANOVA for statistical significance. The null hypothesis was rejected for P < 0.05.
RESULTS
Neutrophil mobility was decreased in MyD88 knockout (KO) and KO→WT chimeric mice.
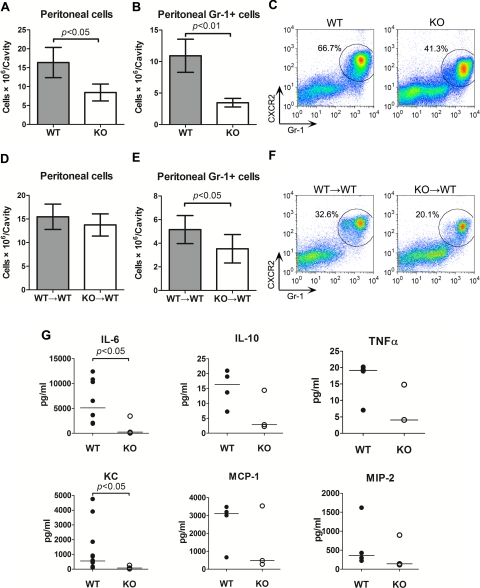
We examined the role of MyD88 signaling in neutrophil migratory function in an in vivo model of neutrophil migration. Thioglycollate was used to elicit peritonitis and neutrophil migration into the peritoneal space. As illustrated in Fig. 1, A and B, there was a marked neutrophil migration to the peritoneal space after thioglycollate injection in WT mice. Neutrophil migration was markedly decreased in MyD88−/− mice compared with WT mice (10.9 ± 1.5 × 106 vs. 3.5 ± 0.4 × 106, 68% reduction) with a decrease in the percentage of neutrophils in the peritoneal cells (66.8 ± 0.2% vs. 41.6 ± 1.7%) as gated on Gr-1 and CXCR2 in flow cytometry. To dissect the role of MyD88 signaling in bone marrow-derived cells in controlling neutrophil migration, we examined neutrophil peritoneal recruitment in the chimeric MyD88-deficient mice. Compared with WT→WT control mice, KO→WT chimeric mice had 33% reduction in neutrophil recruitment (Fig. 1E). Of note, the reduction in peritoneal neutrophil recruitment in KO or KO→WT mice was not due to reduced numbers of blood neutrophils in these mice because no difference was observed in the numbers of total neutrophils among the four groups of mice (WT, 1,780 ± 77/μl; KO, 1,303 ± 157/μl; WT→WT, 1,513 ± 296/μl; KO→WT, 1,011 ± 35/μl; n = 3–5). Taken together, these data suggest that MyD88 signaling, both that of bone marrow-derived and resident parenchymal cells, plays an important role in maintaining normal neutrophil migratory function in vivo.
Fig. 1.
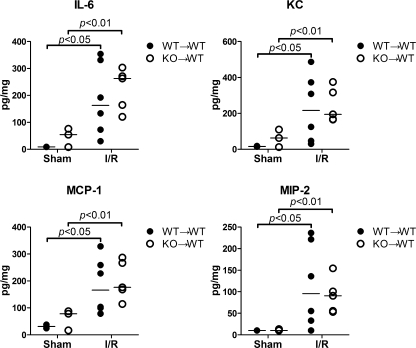
Knockout (KO) and KO→wild-type (KO→WT) mice have impaired neutrophil mobility and reduced peritoneal cytokine/chemokine levels. Mice were injected intraperitoneally with thioglycollate. Ten hours after the injection, 3 ml of normal saline was injected and mixed well. The peritoneal lavage fluid and leukocytes were isolated and analyzed. A: total numbers of cells recruited into the peritoneal cavity of WT and KO mice. B: total neutrophils recruited into the peritoneal cavity of WT and KO mice. Each error bar represents mean ± SD of 3 mice. C: a representative example of flow cytometry plots of peritoneal neutrophils from WT and myeloid differentiation factor 88 (MyD88)-deficient mice (MyD88−/−). The cells were gated on CXCR2 and Gr-1. The cells within the circle represent CXCR2+/Gr-1+ neutrophils, and the percentage of neutrophils is shown. D: total numbers of cells recruited into the peritoneal cavity of the chimeric mice. E: total neutrophils recruited into the peritoneal cavity of the chimeric mice. Each error bar represents mean ± SD of 3 mice. F: a representative example of flow cytometry plots of peritoneal neutrophils from WT→WT and KO→WT chimeric mice. G: peritoneal lavage was analyzed for cytokine production using Luminex multiplex immunoassay. The bars in each dot plot represent median values of the measured cytokines. Some cytokine values were overlapping. n = 4–7. MCP-1, monocyte chemoattractant protein 1; MIP-2, macrophage inflammatory protein 2.
MyD88−/− mice had reduced peritoneal cytokine production.
In vivo neutrophil migration is critically affected by chemokine levels at the site of infection or injury. To test whether or not the attenuated peritoneal neutrophil migration observed in MyD88−/− mice could be the result of decreased chemokine production in the peritoneal space, we measured the chemokine and cytokine levels of peritoneal lavage after intraperitoneal injection of thioglycollate. As indicated in Fig. 1G, KC, a potent neutrophil chemoattractant and a CXC ligand for CXCR2, and IL-6, an important proinflammatory cytokine, were significantly lower in the peritoneal lavage of MyD88−/− mice compared with that in WT mice, whereas the levels of monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 2 (MIP-2), two chemoattractants for macrophages and neutrophils, respectively, and the cytokines TNF-α and IL-10, were not significantly different between WT and MyD88−/− mice.
CXCR2 expression on the peritoneal neutrophils was downregulated in MyD88 KO and KO→WT chimeric mice.
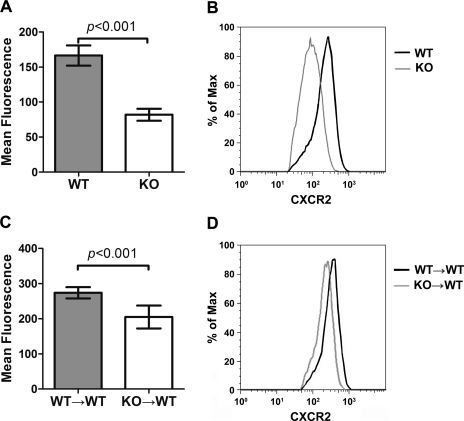
In addition to chemokine gradients, CXCR2 expression on neutrophils is also critical for normal neutrophil migratory function (31). We examined the impact of MyD88 deletion on CXCR2 expression on both bone marrow neutrophils and peritoneal neutrophils. MyD88−/− mice had the same levels of CXCR2 expression on their bone marrow neutrophils compared with WT mice (data not shown). This was true for both the control mice that received no treatment and the mice with thioglycollate-induced peritonitis. In contrast, there was a marked reduction (>60%) in the peritoneal neutrophil CXCR2 expression in MyD88−/− mice compared with WT mice (Fig. 2, A and B). Interestingly, compared with WT→WT control, the peritoneal neutrophil CXCR2 expression in KO→WT mice was also significantly decreased, but to a lesser degree (30%) (Fig. 2, C and D). Taken together, these data suggest that MyD88 signaling, both that of bone marrow-derived cells and of peritoneal resident cells, plays an important role in maintaining CXCR2 expression on neutrophils during active inflammation. The reduced CXCR2 expression may explain in part the attenuated neutrophil mobility in the MyD88−/− mice.
Fig. 2.
KO and KO→WT mice have decreased neutrophil CXCR2. A: CXCR2 expression of neutrophils recruited to the peritoneum in WT and MyD88−/− mice. The y-axis represents CXCR2 expression as measured by mean fluorescence. Each error bar represents mean ± SD of 3–6 mice. B: a representative example of FACS plots of neutrophil CXCR2 expression in WT (red) or MyD88−/− mice (green). C: CXCR2 expression on neutrophils recruited to the peritoneal space in WT→WT and KO→WT mice. Each error bar represents mean ± SD of 3–6 mice. D: a representative example of FACS of neutrophil CXCR2 expression in WT→WT (red) and KO→WT (green) mice.
MyD88 signaling modulates KC-induced CXCR2 downregulation in neutrophils.
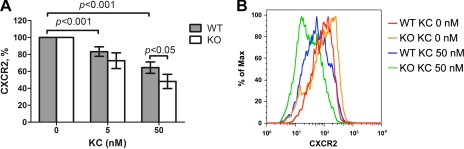
Our pilot study established that bone marrow-derived neutrophils from WT and MyD88−/− mice had the same CXCR2 expression and the same in vitro chemotaxis to KC (data not shown). We hypothesized that the differential CXCR2 expression on the peritoneal neutrophils in WT and MyD88−/− mice had developed after the neutrophils were exposed to cytokines and chemokines in the inflammatory site of the peritoneal space and that MyD88 signaling could modulate the cytokine (such as KC)-mediated CXCR2 downregulation. Cytokines (2, 21) and phagocytosis (12) are known to downregulate the plasma membrane expression of neutrophil CXCR2. We have also reported that marked CXCR2 downregulation occurs in infectious peritonitis (40). To determine whether MyD88 signaling could modulate this process, we tested KC-induced CXCR2 downregulation in both WT and MyD88−/− neutrophils in vitro. As illustrated in Fig. 3, incubation with KC induced a dose-dependent CXCR2 downregulation in neutrophils isolated from WT bone marrow. However, compared with the WT neutrophils, MyD88−/− neutrophils appeared more prone to the KC-induced downregulation and had significantly lower CXCR2 expression after exposed to 50 nM KC.
Fig. 3.
MyD88 deficiency enhances the KC-induced downregulation of neutrophil CXCR2 expression. Bone marrow-derived neutrophils from both WT and MyD88−/− mice were incubated with or without the indicated KC concentrations at 37°C for 30 min. Cells were stained and analyzed in flow cytometry and gated on Gr-1 and CXCR2. A: accumulated data of neutrophil CXCR2 expression presented as the percentage of the control cells without KC treatment. Each error bar represents mean ± SD of 4 separate neutrophil preparations. B: a representative plot of flow cytometry illustrating neutrophil CXCR2 expression in the control cells and the cells treated with 50 nM KC from WT and MyD88−/− mice.
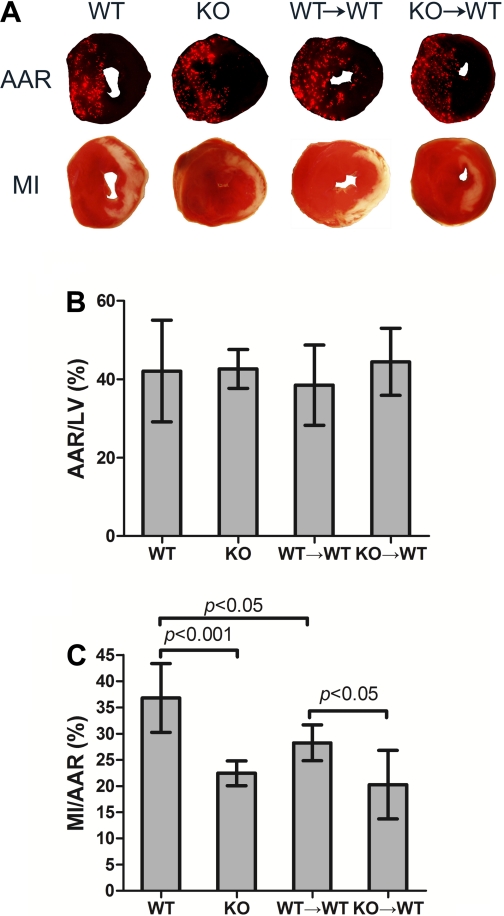
KO→WT mice had smaller MI size compared with WT→WT mice after I/R.
To determine the role of circulating MyD88 in the development of myocardial ischemic injury, we subjected the four groups of mice, i.e., WT, KO, WT→WT, and KO→WT, to 30 min of ischemia and 24 h of reperfusion (I/R) and examined infarct sizes. Figure 4A shows representative photographs of AAR and MI. The ratios of AAR to left ventricle (LV) were similar among the groups (Fig. 4B). As illustrated in Fig. 4C, similar to MyD88−/− (KO) mice, WT mice transplanted with MyD88−/− bone marrow cells (KO→WT) had markedly reduced MI size compared with WT mice (20 ± 2% vs. 37 ± 2%, P < 0.01) or with the WT→WT controls (28 ± 1%, P < 0.05), suggesting that MyD88 signaling in bone marrow-derived circulating cells may contribute to myocardial injury during I/R. Surprisingly, the infarct sizes of WT→WT mice were significantly smaller than that of WT mice (28 ± 1% vs. 37 ± 2%, P < 0.05) (Fig. 4C), which may suggest that irradiation and/or bone marrow reconstitution confers protective effects in this group of chimeric animals. Both bone marrow cell transfusion (28, 36) and irradiation (22) have been reported to confer protective benefits against ischemic injury. To dissect the effect of bone marrow cell transfusion from that of irradiation, we transfused WT mice that received no irradiation with WT bone marrow cells and 8 wk later examined the MI sizes of these mice. We found that the WT→WT mice (without irradiation) had similar MI sizes compared with the WT mice (23.0 ± 1.8% vs. 28.8 ± 3.1%, n = 5–7, P > 0.05). These data suggest that bone marrow transfusion per se does not confer cardiac protection against I/R injury and that the MI reduction observed in WT→WT mice after irradiation and bone marrow transfusion may be due to irradiation.
Fig. 4.
KO→WT mice have smaller myocardial infarction (MI) size compared with WT→WT mice. Mice were subjected to 30 min of ischemia and 24 h of reperfusion. At the end of reperfusion, animals were euthanized, and area-at-risk (AAR) and MI were analyzed. A: representative pictures of AAR and MI from the 4 groups of mice. B: cumulative data of AAR/left ventricle (LV). C: cumulative data of MI/AAR. Each error bar represents mean ± SD of 6–9 mice.
Myocardial cytokine production after I/R is independent of MyD88 signaling in bone marrow-derived circulating cells.
Myocardial inflammatory responses such as cytokine production occur during I/R and are known to contribute to myocardial injury as well as dysfunction. We tested whether MyD88 signaling in bone marrow-derived circulating cells was essential in myocardial cytokine and chemokine production after I/R. The control WT→WT chimeric mice had very robust production of IL-6, KC, MCP-1, and MIP-2 proteins in the heart following I/R. To our surprise, there was no significant difference in the cytokine levels between WT→WT and KO→WT mice (Fig. 5) or between the two groups of chimeric mice and WT mice (data not shown). These data strongly suggest that myocardial cytokine expression after I/R is independent of MyD88 signaling in bone marrow-derived circulating cells.
Fig. 5.
Bone marrow MyD88 deficiency has no impact on myocardial cytokine levels during ischemia-reperfusion (I/R). Both WT→WT and KO→WT chimeric mice were subjected to sham operation or I/R. The hearts were isolated, and myocardial cytokines were measured using Luminex multiplex immunoassay. The bars in each dot plot represent median values of the measured cytokines. Some cytokine values were overlapping. n = 5–6.
MyD88−/− mice had attenuated myocardial neutrophil recruitment after I/R.
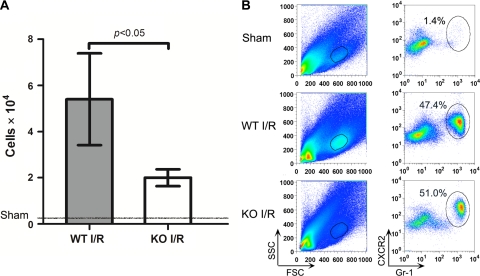
Neutrophils play a critical role in acute myocardial injury during I/R. Neutrophil depletion leads to reduced myocardial injury after I/R (13, 30, 32). To examine whether MyD88 deficiency has any impact on myocardial neutrophil recruitment after I/R, we used flow cytometry to quantify the number of neutrophils recruited into the myocardium in response to I/R in WT and MyD88−/− mice. As indicated in Fig. 6, I/R induced a marked increase in Gr-1+ neutrophil recruitment into the myocardium, whereas the sham-operated mice had minimal neutrophils present in their myocardium. Compared with WT mice, MyD88−/− mice had a marked reduction in myocardial Gr-1+ neutrophil recruitment in response to I/R injury (5.4 ± 1.2 × 104 vs. 2.0 ± 0.2 × 104). Gr-1+ neutrophils were further confirmed with flow cytometry gated for Ly-6G, which is more specific for neutrophils (see Supplemental Material for details).
Fig. 6.
MyD88−/− mice have marked attenuation in myocardial neutrophil recruitment after I/R. Twenty-four hours after 60 min of left anterior descending coronary artery (LAD) ligation, the hearts were isolated, perfused, and digested. After removal of the large cardiomyocytes through filtration, 50% of total cells were loaded onto flow cytometry and gated on Gr-1 and CXCR2. A: total Gr-1+ cells as measured by flow cytometry. Each error bar represents mean ± SD of 4 mice. A small number of neutrophils were recovered from the sham-operated hearts as indicated by the line. B: a representative example of flow cytometry plots of myocardial infiltrating cells from sham, WT-I/R, and KO-I/R mice. FSC, forward scatter; SSC, side scatter.
Trif signaling has no effect on myocardial neutrophil recruitment in vivo and CXCR2 downregulation in vitro.
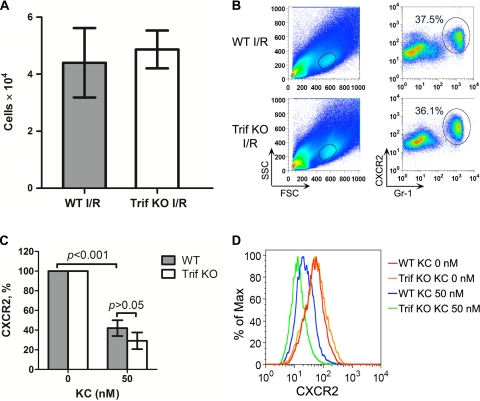
To determine whether or not myocardial neutrophil recruitment after I/R and CXCR2 expression modulation were specific to MyD88-dependent pathway, we examined the effect of Trif-dependent signaling pathway. As shown in Fig. 7, A and B, systemic Trif-deficiency did not impact on myocardial neutrophil recruitment following I/R as demonstrated by Gr-1+-gated flow cytometry. In vitro, isolated Trif−/− neutrophils responded to KC treatment with a marked downregulation in CXCR2 expression, but to the similar degree as WT neutrophils (Fig. 7, C and D).
Fig. 7.
Toll-interleukin-1 receptor (TIR)-domain-containing adaptor protein-inducing interferon-β-mediated transcription factor (Trif) deficiency has no impact on myocardial neutrophil recruitment and KC-induced CXCR2 downregulation. Twenty-four hours after 60 min of LAD ligation, the isolated hearts were perfused and digested. After removal of the large cardiomyocytes, 50% of total cells were loaded onto flow cytometry and gated on Gr-1 and CXCR2. A: total Gr-1+ cells as measured by flow cytometry from the hearts subjected to I/R. Each error bar represents mean ± SD of 3 mice. B: a representative example of flow cytometry plots of myocardial infiltrating cells from WT-I/R and Trif-KO-I/R mice. C: accumulated data of neutrophil CXCR2 expression presented as the percentage of the control cells without KC treatment. Each error bar represents mean ± SD of 3 separate neutrophil preparations. D: a representative plot of flow cytometry illustrating neutrophil CXCR2 expression in the control cells and the cells treated with 50 nM KC from WT and Trif-KO mice.
DISCUSSION
Several lines of evidence suggest that innate immune system components such as TLRs may play an essential role in myocardial inflammation and injury during I/R (1, 10, 14, 29). However, the underlying mechanisms of the pathological process remain incompletely understood. In this study, we investigated how MyD88 signaling modulated neutrophil function in two models of tissue inflammation, i.e., thioglycollate-elicited peritonitis and transient ischemia-induced myocardial inflammation, and its impact on myocardial injury induced by I/R. We found that neutrophil mobility was severely impaired in MyD88−/− mice and in mice deficient of MyD88 in their bone marrow-derived circulating cells. MyD88−/− mice had significantly lower CXCR2 expression on their neutrophils that migrated into the peritoneal inflammatory loci in vivo, and their neutrophils were more prone to KC-induced CXCR2 downregulation in vitro. MyD88 chimeric KO→WT mice, which lacked MyD88 in bone marrow-derived circulating cells but maintained normal MyD88 signaling in the heart, had significantly smaller MI sizes compared with WT→WT controls. In contrast, Trif-deficient mice had similar levels of neutrophil infiltration in their hearts after I/R compared with WT mice and had similar levels of neutrophil CXCR2 expression as WT after KC treatment. These data suggest that MyD88 signaling, particularly that of bone marrow-derived circulating cells, plays a critical role in maintaining normal neutrophil migratory function and CXCR2 expression and contributes to the development of myocardial I/R injury.
Our study demonstrates that MyD88 signaling plays an important role in neutrophil migratory function, which is an intricate part of the inflammatory process. MyD88−/− mice had severely impaired neutrophil mobility into the peritoneal space. MyD88 deficiency in bone marrow cells (i.e., in KO→WT mice) also led to impairment of neutrophil mobility, although to a lesser degree, compared with MyD88−/− mice. These data clearly suggest that neutrophil mobility is dependent on the MyD88 signaling of both the recruited bone marrow-derived circulating cells and the peritoneal resident cells. In response to inflammatory stimuli, the peritoneal resident cells synthesize and secrete chemokines into the peritoneal space, which attract circulating neutrophils into the inflammatory site. The chemoattractant effect is known to be dependent on the chemokine gradient between the blood and the site of inflammation and injury. The markedly decreased peritoneal level of KC and IL-6 in MyD88−/− mice may explain in part the observed reduction in neutrophil migration into peritoneal space. Plasma membrane CXCR2 expression is also critical for neutrophil migratory function. Our finding that MyD88−/− or KO→WT MyD88 chimeric mice had significant reduction in CXCR2 expression on their peritoneal neutrophils strongly indicates that MyD88 signaling, either in bone marrow-derived cells or in peritoneal resident cells, controls neutrophil migratory function by maintaining neutrophil CXCR2 expression. MyD88 deficiency leads to reduced neutrophil CXCR2 expression and hence impaired neutrophil migratory function. We speculate that the impairment of neutrophil function is probably responsible for the marked reduction in myocardial neutrophil recruitment during I/R in MyD88−/− mice.
Our previous study has established that global MyD88 deletion confers cardiac protection against I/R injury and attenuates myocardial inflammation (14). Because myocardial inflammation is an intricate part of I/R injury that involves both myocardial tissue as well as circulating inflammatory cells, we sought to dissect the contribution of myocardial and circulating MyD88 to I/R injury. We generated the chimeric mice that specifically lacked MyD88 expression in their bone marrow-derived hematopoietic cells but maintained normal MyD88 expression in the heart. Similar chimeric models have been validated and widely used to determine the role of hematopoietic versus parenchymal TLR-MyD88 signaling in the host immune response to invading pathogens or in noninfectious tissue injury (1, 3–5, 26). The finding that KO→WT mice have significant reduction in MI sizes compared with WT→WT mice suggests that MyD88 signaling in circulating cells plays an important role in the development of I/R injury. This is consistent with our previous finding that MyD88 deficiency confers cardiac protection against I/R injury only in vivo but not in isolated heart (14). The result is also in agreement with a recent study by Arslan et al. (1), who have demonstrated that myocardial infarction size after I/R is determined by circulating leukoctyte TLR-2. It is noteworthy that while MI sizes of KO→WT mice were reduced to the level of KO mice, a 46% reduction compared with WT, part of the cardiac protective benefit observed in the KO→WT chimeric mice might be due to irradiation since the WT→WT chimeric controls also had modest but significant reduction in MI sizes compared with the WT control mice. Both irradiation and bone marrow transplantation have been shown to protect against ischemic injury. Casium-137 irradiation has been recently found to have a protective preconditioning effect against kidney I/R injury via superoxide production and enhanced heat shock protein-27 expression (22). A number of studies in animals and human have demonstrated that intravenous transfusion or direct myocardial injection of bone marrow or enriched bone marrow-derived stem cells markedly improves LV function after I/R injury (28, 34, 36). However, in our studies, while WT→WT chimeric mice exhibited reduced MI sizes compared with WT animals, bone marrow transfusion without prior irradiation in WT mice did not achieve any MI reduction after I/R. These data suggest that bone marrow transfusion per se does not confer any protection and that the observed cardiac benefit in the WT→WT chimeric mice is probably due to irradiation, rather than bone marrow transfusion. Taken together, these chimeric studies demonstrate that lack of MyD88 signaling in bone marrow-derived circulating cells results in a significant reduction in myocardial MI and that irradiation may confer an additional cardiac protection against I/R injury in the chimeric mice.
Neutrophil recruitment and cytokine production are an important part of inflammatory process. Following ischemia and during the reperfusion phase, circulatory neutrophils are recruited to the myocardium in response to a number of proinflammatory parameters such as adhesion molecules and cytokines/chemokines. The recruited neutrophils may contribute to myocardial injury and cardiac dysfunction. IL-6 is a potent proinflammatory cytokine and has a direct effect on cardiomyocyte survival, remodeling, and contractile function (23, 33), whereas KC, MIP-2, and MCP-1 are chemoattractants important for neutrophil and macrophage recruitment into injured tissues. The lack of a significant difference in myocardial chemokine expression following I/R between WT→WT and KO→WT chimeric mice (and between WT and the chimeric mice, data not shown) suggests that the myocardial cytokine/chemokine production in response to I/R is independent of MyD88 signaling in bone marrow-derived hematopoietic cells and is probably mainly determined by myocardial MyD88 expression. Given the indifferent myocardial cytokine expression between the chimeric mice and the substantial reduction of MI size in KO→WT mice compared with WT→WT, we conclude that enhanced myocardial cytokine levels contribute little to the observed difference in MI sizes between these two groups of chimeric mice. Proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, are not constitutively expressed in the normal heart. In response to myocardial injury such as I/R-induced MI or mechanic stress, there is robust upregulation of myocardial cytokines that are synthesized not only by infiltrated immune cells but also by cardiomyocytes (27). This cytokine production may represent an important form of acute adaptive response in the heart in the setting of I/R and provide an intrinsic protection in the heart (23, 33).
It is noteworthy that some bone marrow mesenchymal stem cells (MSC) might have been transplanted into the receipt mice and could become nonhematopoietic cells residing in the heart and other parenchymal tissues. The possible contribution of donor bone marrow-derived nonhematopoietic MyD88 to I/R injury cannot be ruled out and needs to be further investigated. However, the observation that WT→WT and KO→WT chimeric mice have the same myocardial cytokine production seems to suggest that at least those possible MSC-derived noncardiomyocytes have minimal contribution to the myocardial inflammation after I/R injury.
It is important to note that the neutrophil migratory function in the ischemic heart and the KC-mediated modulation of neutrophil CXCR2 expression appear to be highly dependent on and specific to MyD88 signaling. Trif, a key adaptor protein unique to TLR-3 and TLR-4 signaling pathways, seems to have little role in these processes since compared with WT mice, Trif-deficient mice have similar levels of myocardial neutrophil recruitment after I/R in vivo and the CXCR2 expression on isolated neutrophils in vitro.
Of note, thioglycollate-induced peritonitis is a widely used model to measure the migratory function of monocytes and neutrophils in response to inflammation (11, 16, 35, 39). The model is clearly different in many ways from I/R-induced myocardial inflammation, such as the cytokine profiles, the kinetics and pattern of inflammatory cell recruitment, and the causes of inflammation and injury. Thus, the peritonitis model was not used as a measure for leukocyte infiltration in ischemic heart, but rather as a model to provide the mechanistic insights into how neutrophil functions were modulated. To this end, the peritonitis model provides some valuable information on the role of MyD88 signaling in regulating the neutrophil migratory function and chemokine expression.
In summary, we have demonstrated that MyD88 signaling is critical for maintaining normal neutrophil migratory function and CXCR2 expression and that MyD88 signaling in bone marrow-derived circulating cells contributes to the development of ischemic myocardial injury.
GRANTS
This work was supported by National Institutes of Health Grant GM-080906 and American Heart Association Grant 0755890T.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Klemen Strle and Shiqian Shen for help with the Luminex Multiplex Immunoassay. We appreciate Dr. Hui Zheng of the Massachusetts General Hospital Biostatistics Center for the advice on statistical analysis.
REFERENCES
- 1.Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 121: 80–90, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Asagoe K, Yamamoto K, Takahashi A, Suzuki K, Maeda A, Nohgawa M, Harakawa N, Takano K, Mukaida N, Matsushima K, Okuma M, Sasada M. Down-regulation of CXCR2 expression on human polymorphonuclear leukocytes by TNF-alpha. J Immunol 160: 4518–4525, 1998 [PubMed] [Google Scholar]
- 3.Binck BW, Tsen MF, Islas M, White DJ, Schultz RA, Willis MS, Garcia JV, Horton JW, Thomas JA. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am J Physiol Heart Circ Physiol 288: H577–H583, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bultinck J, Brouckaert P, Cauwels A. The in vivo contribution of hematopoietic cells to systemic TNF and IL-6 production during endotoxemia. Cytokine 36: 160–166, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 25: 1788–1796, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao W, Shen Y, Li L, Rosenzweig A. Importance of FADD signaling in serum deprivation- and hypoxia-induced cardiomyocyte apoptosis. J Biol Chem 277: 31639–31645, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chao W, Shen Y, Li L, Zhao H, Meiler SE, Cook SA, Rosenzweig A. Fas-associated death-domain protein inhibits TNF-α mediated NF-κB activation in cardiomyocytes. Am J Physiol Heart Circ Physiol 289: H2073–H2080, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem 280: 21997–22005, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Dong F, Khalil M, Kiedrowski M, O'Connor C, Petrovic E, Zhou X, Penn MS. Critical role for leukocyte hypoxia inducible factor-1alpha expression in post-myocardial infarction left ventricular remodeling. Circ Res 106: 601–610, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Doroshenko T, Chaly Y, Savitskiy V, Maslakova O, Portyanko A, Gorudko I, Voitenok NN. Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood 100: 2668–2671, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schonbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol Heart Circ Physiol 251: H314–H323, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Zhao H, Xu X, Buys ES, Raher MJ, Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U, Chao W. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol 295: H1311–H1318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA. A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. J Mol Cell Cardiol 49: 280–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci 27: 474–482, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. TLR signaling. Cell Death Differ 13: 816–825, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 167: 5887–5894, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Khandaker MH, Mitchell G, Xu L, Andrews JD, Singh R, Leung H, Madrenas J, Ferguson SS, Feldman RD, Kelvin DJ. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood 93: 2173–2185, 1999 [PubMed] [Google Scholar]
- 22.Kim J, Park JW, Park KM. Increased superoxide formation induced by irradiation preconditioning triggers kidney resistance to ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 296: F1202–F1211, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol 65: 81–101, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest 61: 661–670, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev 173: 89–97, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24: 79–91, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann NY Acad Sci 938: 221–229, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67: 1016–1023, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7: 151–185, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka M, Brooks SE, Richard VJ, FitzHarris GP, Stoler RC, Jennings RB, Arfors KE, Reimer KA. Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation 87: 526–535, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Taqueti VR, Mitchell RN, Lichtman AH. Protecting the pump: controlling myocardial inflammatory responses. Annu Rev Physiol 68: 67–95, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100: II247–II256, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood 111: 42–49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 42: 441–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118: 1837–1847, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med 38: 1335–1342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.