Abstract
Previous clinical evaluation of FK506 in renal transplantation has demonstrated equivalent patient and graft survival when compared with cyclosporine-based regimens. However, lower steroid and anti-hypertensive medication requirements and lower serum cholesterol levels have been seen in patients receiving FK506. In August, 1991, a prospective, randomized trial was begun, comparing FK506/prednisone with FK506/azathioprine/prednisone. Two-hundred-and-four adults were entered into this trial between August 1, 1991, and October 11, 1992. The mean recipient age was 43.8 ± 13.7 years, with a range of 17.6–78.0 years. Sixty-one (30%) recipients received a 2nd, 3rd or 4th transplant, while 35 (17%) had a PRA greater than 40% at the time of transplant. Thirty-three (16%) of the transplants were in recipients over 60 years of age, Thirteen percent of the kidneys were from living donors; 13% of the cadaveric kidneys were from pediatric donors less than 3 years of age and were transplanted en bloc. The mean cold ischemia time was 31.4 ±8.4 hours, and the mean donor age was 34 ±2.10 years, with a range from 4 months to 75 years. With a mean follow-up of 9 ± 4 months, the 1-year actuarial patient survival is 93%; for the two-drug group it is 95%, and for the three-drug group it is 91 % (p = NS). One-year actuarial graft survival is 86%; in the two-drug group it is 90%, while in the three-drug group it is 82% (p = NS). The mean serum creatinine and BUN are 1.85 ± 0.76 mg/dl and 30 ± 14 mg/dl; the values are not significantly different between the two- and three-drug groups. Rejection was seen in 45% of patients, 51% in the two-drug and 39% in the three-drug group (p = 0.09). In cadaveric recipients, more rejection was seen in the two-drug group (58%) than in the three-drug group (39%; p < 0.02: 24 (12%) of patients required OKT3 or ATGAM® for rejection: 24 (12%) had cytomegalovirus; an equal incidence was seen in both groups. New onset diabetes was seen in 14% of patients; there was a higher incidence in the two-drug (20%) than in the three-drug (8%) group (p < 0.03). The incidence of PTLD was 1% (2 patients). Crossover between the two limbs was seen commonly: 26/25%) of the patients in the two-drug group required the addition of azathioprine, while 46 (45%) of the patients in the three-drug group required discontinuation of azathioprine (usually because of a falling white blood cell count or hepatic dysfunction). Sixty-five (32%) patients are off steroids, while 88 (43%) patients are not taking any antihypertensive medications. The mean serum cholesterol is 193 ± 53 mg/dl. These data confirm earlier reports about the efficacy of FK506 in renal transplantation. The benefit of azathioprine is unclear, with no improvement in patient and graft survival and a higher crossover rate, but with less rejection in certain subgroups and less diabetes.
Keywords: kidney transplantation, FK 506
Introduction
The new immunosuppressive drug FK-506 has been used as the primary agent after transplantation of nearly all organs, including the liver (1), kidney (2), heart (3), lung (4), small intestine (6) and pancreatic islets (7). The early experiences in renal transplantation have been encouraging (2, 7–12). A large comparative experience between cyclosporine and FK-506 revealed equivalent patient and graft survival, but improved results with FK-506 in terms of secondary outcomes – lower steroids and antihypertensive requirements, and lower serum cholesterol levels (8). On the basis of these earlier studies, a prospective, randomized trial was begun to evaluate two different FK-506-based regimens, with and withoug azathioprine. A preliminary report of this trial, while showing promising results, was limited by the short follow-up (9); 1-yr actuarial data are now available for presentation.
Material and methods
Entry criteria
Between 1 August, 1991, and 11 October, 1992, 204 patients were entered into a randomized trial comparing FK-506 and prednisone with FK-506, azathioprine, and prednisone. Adult patients undergoing kidney transplantation only who agreed to participate in the trial were eligible. Pediatric patients, patients receiving another organ concomitanly (liver or pancreatic islets), or patients who refused were not entered into the trial. The protocol was reviewed and approved by the Institutional Review Board of the University of Pittsburgh.
Recipient characteristics
The mean recipient age was 43.8 ± 13.7 yr, with a range of 17.6–78 yr (Table 1). Sixty-one (30%) patients were undergoing retransplantation, receiving their 2nd (41–20%), 3rd (15–7%), or 4th (5–3%) transplant. Thirty-seven (18%) patients had a panel-reactive antibody (PRA) greater than 40% at the time of transplantation. Thirty-three (16%) of the recipients were over 60 yr of age. Thirty-four (17%) recipients were either Black (28–14%), Asian (4–2%), or Hispanic (2–1%). The causes of end stage renal disease are listed in Table 2; diabetes mellitus was the most common indication for transplantation. Eight (4%) patients had previously undergone orthotopic liver transplantation.
Table 1.
Recipient characteristics
| FK506/pred | FK506/aza/pred | Overall | |
|---|---|---|---|
| Patients | 102 | 102 | 204 |
| Mean recipient age | 42.1 ± 13.1 yr | 45.4 ± 14.1 yr | 43.8 ± 13.7 yr |
| (Range) | (17.6–69.6) | (18.1–78.0) | (17.6–78.0) |
| 1st transplant | 73 (72%) | 70 (69%) | 143 (70%) |
| Retransplant | 29 (29%) | 32 (31%) | 61 (30%) |
| PRA ≥ 40% | 17 (17%) | 20 (20%) | 37 (18%) |
| * Age ≥ 60 years | 11 (11%) | 22 (22%) | 33 (16%) |
| Non-black | 89 (87%) | 87 (85%) | 176 (86%) |
| Black | 13 (13%) | 15 (15%) | 28 (14%) |
p <0.04.
Table 2.
Causes of end-stage renal disease
| FK506/pred | FK506/aza/pred | Overall | |
|---|---|---|---|
| Diabetes mellitus | 29 (28%) | 21 (21%) | 50 (25%) |
| Glomerulonephritis | 18 (18%) | 13 (13%) | 31 (15%) |
| Hypertension | 11 (11%) | 16 (16%) | 27 (13%) |
| Polycystic kidney disease | 7 (7%) | 10 (10%) | 17 (8%) |
| Focal segmental glomerulosclerosis | 8 (8%) | 5 (5%) | 13 (6%) |
| Pyelonephritis | 5 (5%) | 3 (3%) | 8 (4%) |
| Systemic lupus erythematosis | 3 (3%) | 6 (6%) | 9 (4%) |
| IgA nephropathy | 0(0%) | 5 (5%) | 5 (2%) |
| Hemolytic-uremic syndrome | 2 (2%) | 1 (1%) | 3 (2%) |
| Alport’s | 2 (2%) | 1 (1%) | 3 (2%) |
| Unknown | 8 (8%) | 6 (6%) | 14 (7%) |
| Other | 9 (9%) | 15 (14%) | 24 (12%) |
Donor characteristics
The mean donor age was 34.3 ± 20.1 yr, with a range of 0.3–75 yr (Table 3). There were 178 (87%) cadaveric, and 26 (13%) living donors. Twenty-four (13%) of the cadaveric kidneys were from pediatric donors under the age of 3 yr and were transplanted en bloc.
Table 3.
Donor characteristics
| FK506/pred | FK506/aza/pred | Overall | |
|---|---|---|---|
| Mean donor age (yrs) | 34.2 ± 19.4 | 34.3 ± 20.8 | 34.3 ± 20.1 |
| Range | 0.3–69 | 0.3–75 | 0.3–75 |
| Mean cold Ischemia time (HRS) | 31.6 ± 8.3 | 31.3 ± 8.7 | 31.4 ± 8.4 |
| Cadaver | 84 (82%) | 94(92%) | 178 (87%) |
| * Living donor | 18 (18%) | 8 (8%) | 26 (132) |
| En bloc | 10 (12%) | 14 (15%) | 24 (13%) |
p<0.021.
The mean cold ischemia time was 31.4 ± 8.4 h. A breakdown of the HLA matches and mismatches is shown in Table 4. There were 7 (3%) six-antigen matches and 13 (6%) zero-antigen mismatches.
Table 4.
| Ag | Antigen mismatching |
||
|---|---|---|---|
| FK506/pred | FK506/aza/pred | Overall | |
| 0 | 8 (8%) | 5 (5%) | 13 (6%) |
| 1 | 8 (8%) | 5 (5%) | 13 (6%) |
| 2 | 24 (24%) | 24 (24%) | 48 (24%) |
| 3 | 31 (30%) | 31 (30%) | 62 (30%) |
| 4 | 18 (18%) | 22 (22%) | 40 (20%) |
| 5 | 10 (10%) | 12 (12%) | 22 (11%) |
| 6 | 3 (3%) | 3 (3%) | 6 (3%) |
| 102 | 102 | 204 | |
| Antigen matching |
|||
| Ag | FK506/pred | FK506/aza/pred | Overall |
| 6 | 5 (5%) | 2 (25%) | 7 (3%) |
| 5 | 7 (7%) | 1 (1%) | 8 (4%) |
| 4 | 15 (15%) | 13 (13%) | 28 (14%) |
| 3 | 32 (31%) | 35 (34%) | 67 (33%) |
| 2 | 26 (25%) | 26 (25%) | 52 (25%) |
| 1 | 14 (14%) | 20 (20%) | 34 (17%) |
| 0 | 3 (3%) | 5 (5%) | 8 (4%) |
| 102 | 102 | 204 | |
The overall distribution of the patients in the two limbs of the trial was not statistically different with regard to the above parameters, with two exceptions; there were more elderly patients (i.e. over 60 yr of age) in the three-drug group (22% vs. 11% – p < 0.04), and more living donor cases in the two-drug group (18% vs. 8% – p < 0.04).
Immunosuppression – (Table 5)
Table 5.
Immunosuppressive protocol
| FK-506 | Steroid | Azathioprine | |
|---|---|---|---|
| Preop | 0.15 mg/kg/po | Methylprednisolone IV 1000 mg | 3 mg/kg |
| Postop | 0.1 mg/kg IV Continuous infusion | Methylprednisolone IV | 3 mg/kg/d |
| 50 mg IV q 6 h×4 doses | |||
| 40 mg IV q 6 h×4 doses | |||
| 30 mg IV q 6 h×4 doses | |||
| 0.15 mg/kg/po bid when taking po | 20 mg IV q 6 h×4 doses | ||
| 20 mg IV q 12 h×2 doses | |||
| Prednisone 20 mg po qd |
FK-506
FK-506, 0.15 mg/kg was given by mouth to all patients preoperatively. Intravenous FK-506, 0.1 mg/kg/d as a continuous infusion, was started in the recovery room and was continued until the patients had return of gastrointestinal function and were able to tolerate a diet. Oral FK-506 was then given at a dose of 0.15 mg/kg twice a day and then adjusted according to FK-506 levels. An attempt was made to achieve plasma levels of approximately 1.5–2.0 ng/ml during the first few weeks after transplantation, although modification was often necessary because of toxicity or rejection. Target levels were gradually decreased to around 0.5–1 ng/ml or less after the first few months.
Steroids
All patients were given a bolus of 1000 mg of intravenous methylprednisolone in the operating room after induction of anesthesia. Postoperatively, patients received a short steroid recycle of 200–20 mg/d of intravenous methylprednisolone over 6 d. Patients were then maintained on oral prednisone 20 mg/d. Further tapering was individualized.
Azathioprine
Patients randomized to receive azathioprine received 3 mg/kg as an oral dose preoperatively. The initial postoperative dose was 3 mg/kg/d, and further adjustments were made based on the white blood cell count; the usual maintenance dose was 0.5–1.5 mg/kg/d.
Randomization
Random assignment of patients to either FK506/prednisone or FK506/azathioprine/prednisone was done using 1 to 1 allocation via a variable block randomization procedure. The blocks sizes were varied (block sizes = 4 and 8) and were randomly selected with equal probability. The order of assignment within each block was determined by generating a random number between 0 and 1 using a random-number generator and then rearranging the random numbers in ascending order (13). The above procedure was repeated until all patients were randomized.
Statistical analysis
The standard two-sample t-test was used to test differences in means while differences in proportions were tested using Pearson’s chi-square test of association.
Patient survival was calculated from the date of kidney transplantation until death and graft survival from the date of kidney transplantation until graft failure, retransplantation, or patient death. Survival curves were generated using the Kaplan-Meier (product-limit) method and were compared using the generalized Wilcoxon (Breslow) test. A multivariate Cox’s regression analysis was performed to adjust the relative risk of graft failure between the two groups based on age of recipient (over 60 yr) and living donor cases. A stepwise procedure was performed to identify high-risk patients for graft failure using all available information collected. A p-value less than 0.05 was considered statistically significant.
Results
The mean follow-up is 9 ± 4 months.
Patient survival
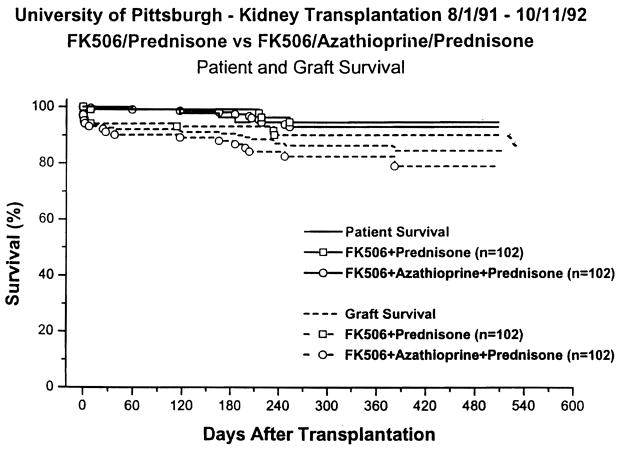
Overall 1-yr actuarial survival was 93%; in the two-drug group it was 95%, and in the three-drug group it was 91 % (Fig. 1; p = 0.35). There were 11 deaths, 4 in the two-drug and 7 in the three-drug group. The causes of death are listed in Table 6.
Fig. 1.
Patient and graft survival.
Table 6.
| Causes of death |
|||
|---|---|---|---|
| FK-506/pred | FK-506/aza/pred | Overall | |
| Infection | 0 | 3 | 3 |
| Cancer | 0 | 2 | 2 |
| Hepatitis | 1 | 0 | 1 |
| Cardiac | 1 | 0 | 1 |
| Disseminated intravascular coagulation | 1 | 0 | 1 |
| Post surgical | 0 | 1 | 1 |
| On dialysis | 1 | 0 | 1 |
| Causes of graft loss |
|||
| FK-506/pred | FK-506/aza/pred | Overall | |
| Rejection | 5 | 4 | 9 |
| Infection | 0 | 5 | 5 |
| Technical | 3 | 2 | 5 |
| Death | 1 | 4 | 5 |
| Disease recurrence | 0 | 2 | 2 |
Graft survival
One-year actuarial graft survival was 86%; in the two-drug group it was 90%, and in the three-drug group it was 82% (Table 7; Fig. 1; p= 0.10). The causes of graft loss are listed in Table 6. Cadaveric recipients had a 1-yr graft survival of 85%, 89% in the two-drug and 82% in the three-drug group (p = 0.19). Living donor recipients had a 1-yr graft survival of 92%, 94% in the two- and three-drug groups, respectively (Fig. 1; p = 0.57). Results in patients undergoing primary of retransplantation; patients with PRA’s below and above 40%; and patients under or over 60 years of age are shown in Table 7. There were no differences either between subgroups or immunosuppressive regimens. Non-blacks had a 1-yr graft survival of 85%, 91 % in the two-drug and 79% in the three-drug group (p = 0.034): black recipients had a 1-yr graft survival of 96%, 92% in the two-drug and 100% in the three-drug groups (p = 0.28) (Table 7).
Table 7.
Graft survival (one year – actuarial)
| FK-506/pred | FK-506/aza/pred | Overall | |
|---|---|---|---|
| All patients | 90% | 82% | 86% |
| Cadaveric | 89% | 82% | 85% |
| Living donor | 94% | 86% | 92% |
| First transplant | 89% | 84% | 87% |
| Retransplant | 93% | 77% | 85% |
| PRA < 40% | 90% | 84% | 87% |
| PRA > 40% | 93% | 75% | 82% |
| Age < 60 | 91% | 82% | 87% |
| Age ≥ 60 | 81% | 83% | 82% |
| Black | 92% | 100% | 96% |
| + Non-black | 91% | 79% | 85% |
| En bloc | 79% | 71% | 76% |
| Other cadaver | 91% | 84% | 87% |
| ATN | 75% | 73% | 74%* |
| ** No ATN | 100% | 87% | 93% |
p = 0.009.
p < 0.00001.
p = 0.034.
Recipients of pediatric en bloc kidneys had a 1-yr graft survival of 76%, with 79% and 71% (p= 0.87) in the two- and three-drug groups; the outcome in the remainder of cadaveric kidneys was 87%, with 91% and 84% 1-yr graft survival in the two- and three-drug groups (p=0.45 between en bloc and other cadaver kidneys).
Acute tubular necrosis (ATN) was seen in 77 (38%) patients; in the two-drug group, there were 42 (41.2%), and in the three-drug group, there were 35 (34.3%) (p=0.312) patients with ATN. ATN was defined either by the absence of function in the first 48 h or by the requirement for dialysis in the 1st wk after transplantation. Overall 1-yr patient survival in the patients with and without ATN was 89% and 95%; overall graft survival was 74% and 93% (p < 0.00001 for differences in graft survival). There was no difference in 1-yr graft survival between the two- and three-drug groups in the ATN group (75% and 73%), but graft survival in the non-ATN group was better in the two-drug group (100% vs. 87%, p=0.009).
In a multivariate analysis performed on the patient and graft survival data, the only significant variable was the presence of ATN as a risk factor for graft survival.
Renal function
The mean serum creatinine was 1.85 ± 0.76 mg/dl. In the two- and three-drug groups it was 1.88 ± 0.70 mg/dl and 1.82±0.85 mg/dl (p=0.299). The median creatinine was 1.7 mg/dl. The mean BUN was 30.3 ± 14.4 mg/dl; in the two- and three-drug groups, it was 30.8± 14.7 mg/dl and 29.7 ± 14.0 mg/dl (p=0.621) (Table 8). There was no significant difference between the two- and three-drug groups in these parameters. The plot of the serum creatinine and BUN over time is shown in Table 9 and 10.
Table 8.
Renal function
| FK-506/pred | FK-506/aza/pred | Overall | |
|---|---|---|---|
| Mean serum creatinine mg/dl | 1.88 ± 0.70 | 1.82 ± 0.85 | 1.85 ± 0.76 |
| Median (mg/dl) | 1.70 | 1.60 | 1.70 |
| Mean BUN (mg/dl) | 31 ± 15 | 30 ± 14 | 30 ± 14 |
Table 9.
| Rejection |
|||
|---|---|---|---|
| FK-506/pred | FK-506/aza/pred | Overall | |
| All patients | 51% | 39% | 45% |
| * Cadaveric | 58% | 39% | 49%+ |
| Living donor | 17% | 25% | 19% |
| PRA < 40% | 46% | 33% | 40%+ |
| PRA ≥ 40% | 80% | 64% | 70% |
| Age < 60 | 53% | 43% | 48% |
| Age ≥ 60 | 36% | 27% | 30% |
| Black | 85% | 60% | 71%+ |
| Non-black | 46% | 36% | 41% |
| Other complications |
|||
| FK-506/pred | FK-506/aza/pred | Overall | |
| Cytomegalovirus | 12% | 12% | 12% |
| PTLD | 1% | 1% | 1% |
| * New onset diabetes | 20% | 8% | 14% |
p = 0.017.
p < 0.05.
p = 0.027.
Table 10.
| FK-506 |
|||
|---|---|---|---|
| FK-506/pred | FK-506/aza/pred | Overall | |
| Dosage (mg/kg/d) | 0.17 ± 0.10 | 0.18 ± 0.10 | 0.17 ± 0.10 |
| Level (ng/ml) | 0.99 ± 0.64 | 1.09 ± 0.88 | 1.0 ± 0.76 |
| Steroid withdrawal |
|||
| FK-506/pred | FK-506/aza/pred | Overall | |
| 31% | 32% | 32% | |
| Anti-hypertensive medications |
|||
| Number | FK-506/pred | FK-506/aza/pred | Overall |
| 0 | 41% | 45% | 43% |
| 1 | 28% | 35% | 32% |
| 2 | 23% | 13% | 18% |
| 3 | 7% | 7% | 7% |
| 4 | 1% | 0% | 1% |
Rejection (Table 9)
Over 90% of rejection episodes were diagnosed by percutaneous renal biopsy. The incidence of rejection was 45%; in the two-drug group it was 51 %, and in the three-drug group it was 39% (p = 0.091). The rejection incidence in cadaveric recipients was 49%; it was 58% (49/84), and 39% (38/94) in the two- and three-drug group (p = 0.017). In living donor cases, the rejection incidence was 19% (p = 0.05, compared to cadaveric transplants); it was 16.7% (3/18), and 25.0% (2/8) in the two- and three-drug groups. The incidence in low and high PRA patients, younger and older recipients, and non-blak and black recipients is shown in Table 8: Rejection was seen more in high PRA patients and blacks, but there were no differences between the two- and three-drug groups in these parameters.
Over 70% of the rejection episodes were steroid-responsive. OKT3® or ATGAM® was used for steroid-resistant rejection, except for 1 patient undergoing retransplantation who received induction OKT3®. Twenty-four (11.8%) patients required OKT3® or ATGAM®; in the two- and three-drug groups, the incidence of OKT3® or ATGAM® requirement was 14.7% and 8.8% (p = 0.192).
Complications – (Table 9)
Cytomegalovirus (CMV)
CMV was seen in 24 (12%) patients; the incidence was identical in the two- and three-drug groups. In general, the presence of either CMV infection or disease was enough to initiate treatment with intravenous gancyclovir. All patients received high-dose acyclovir prophylaxis; seronegative recipients of seropositive kidneys received CMV hyperimmune globulin as well.
Post-transplant lymphoproliferative disorder (PTLD)
PTLD was seen in 2 patients, 1 each in the two- and three-drug groups. The patient in the two-drug group had not been treated for rejection prior to the diagnosis of PTLD; she developed GI lesions, as well as a focus in the renal allograft. The patient in the three-drug group had received steroids for rejection. His PTLD was limited to a few skin lesions. In the 1st patient, the PTLD disappeared with cessation of the immunosuppression; she later experienced rejection, which was reversed with resumption of FK-506 and a short, low-dose steroid recycle. The 2nd patient had a significant decrease in immunosuppression, with prompt disappearance of the skin lesions. Both patients have stable functioning kidneys.
There was 1 case of Kaposi’s sarcoma diagnosed in a skin lesion in the three-drug group. She had returned home overseas and had temporarily been lost to follow-up. This lesion regressed with cessation of FK-506 and a decrease in the prednisone dose.
Diabetes Mellitus
New-onset insulin-dependent diabetes was seen in 20 (13.6%) patients, 14/69 (20.2%) in the two-drug and 6/78 (7.7%) in the three-drug group (p = 0.027). It was able to be discontinued in half of these patients after a reduction in the FK-506 and prednisone dosage.
Immunosuppression
FK-506
The FK-506 dosage and levels decreased over time in a similar manner in the two- and three-drug groups (Table 10). The gradual decrease over time was an individualized clinical decision, and took into account the plasma level, as well as the presence of nephrotoxicity (either clinical or biopsy-proven), neurotoxicity (principally tremors paresthesias of the extremities, and insomnia), infectious complications, new onset diabetes and, occasionally, hair loss.
Steroids – (Table 10)
Prednisone was tapered off completely in 65/204 (32%) of patients, 32 (31.4%) in the two-drug and 33 (32.4%) in the three-drug groups. The overall prednisone dose was not different between the two- and three-drug groups.
Crossover
Azathioprine had to be discontinued in 46 (45%) patients in the three-drug group at one time or another, because of a low white blood cell count or elevated liver function tests. Twenty-six (25%) patients in the double-drug group at one time or another required the addition of azathioprine because of rejection.
Cholesterol
The mean serum cholesterol level was 193 ± 50 mg/dl; it was nearly identical in the two- and three-drug groups.
Antihypertensive medications (Table 10)
Eighty-eight (43%) patients are off antihypertensive medications entirely; in the two- and three-drug groups, there were 42 (41.2%,) and 46 (45%) patients off antihypertension medications.
Discussion
New immunosuppressive agents are generally evaluated in carefully chosen patients and circumstances, i.e. first graft, low PRA, good early function. These ideal circumstances make it possible to assess the potential of the new agents unencumbered by the sort of complicated situations transplant surgeons routinely face. From the outset of our experience with FK-506, a philosophic decision was made not to limit access to the drug to ideal patients and cases. While this made it possible for patients who had failed previous transplants to receive FK-506, it made analysis of the merits of the drug more complicated. In addition, learning to use FK-506 in renal transplantation has been more difficult than in liver transplantation, primarily because of its nephrotoxicity, but also because of the fact that the liver metabolizes FK-506 so quickly that the levels seen by the kidney are lower than those seen by the liver. Our impression has been that the nephrotoxicity of FK-506 has been comparable to that seen with cyclosporine (14).
There has been an unquestionable learning curve with FK-506 in renal transplantation. This is reflected in the 1-yr actuarial graft survival statistics obtained between 1989 and 1991, 74% (8), and those obtained in the current series, 86%. Reasonable results have been obtained with FK-506 and, in our patient population, they are better than what we have seen under cyclosporine-based therapy (15). The ongoing ability to come off steroids in many patients is an additional advantage. Finally, the ability to achieve these results without induction antilymphocyte preparation may have economic consequences; a course of any of these agents can cost over $5000, and we are entering an era where a premium will be placed on trying to decrease costs.
The utility of azathioprine as a third agent was unclear in this study. There was a lower rejection rate in the triple-drug group, in cadaveric recipients, and a lower incidence of new-onset diabetes; it was also necessary to add azathioprine in 25% of the patients randomized to double therapy. However, there was no advantage in patient and graft survival overall, and in fact a worse outcome in graft survival in certain subgroups (non-black recipients and those with immediate allograft function). In addition, a substantial number could not tolerate azathioprine, with a crossover incidence (to the two-drug group) of 45%. Clearly, a subset of patients needs an additional agent, but azathioprine may not be the ideal third drug. It is possible that one of the new investigational agent, such as RS 61443 (16), Brequinar (17), Bredinin (18), or possibly Leflunomide (19), may be more useful.
In this prospectively randomized group of patients, an extensive subgroup analysis was performed to search for significant factors affecting both graft survival and the incidence of rejection. Although an argument could be made that such subgroup analysis is of questionable value, we found it helpful in understanding and interpreting our results.
FK-506 appears to be an efficacious drug for renal transplant patients. Reasonable patient and graft survival can be obtained, even in difficult cases. The agent requires some experience to be used optimally; it is our hope that other centers will learn from ours, so that they have a shorter learning curve when FK-506 is eventually released for general use.
Acknowledgments
We would like to thank Regina Fenton, RN, BSN, CCTC, Loraine Kaminski, RN, Joan Murray RN, BSN, CCTC, Deborah Good RN, BSN, CCTC, Holly Woods RN, CCTC, Marie Hawranko RN, CCTC, Jareen Flohr RN, BSN, Sue Bauder RN, Janice Zagari RN, BSN, and Jennifer Ovesney, RN, BSN, for their help with patient care; Janet Schmelzer for her help with data entry and organization; Dan Dziak for his help with data collection; David Krakosky for his help with graph and slide preparation; Kate Carr for her help with slide preparation; and Karen Blair for her help with typing the manuscript and table and slide preparation.
Footnotes
A prospective, randomized trial of FK-506 in renal transplantation – A comparison between double- and triple-drug therapy.
References
- 1.Fung JJ, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK-506 vs. cyclosporine. Transplantation Proc. 1991;23:6, 2977–2983. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Fung JJ, Jordan M, et al. Kidney transplantation under FK-506. JAMA. 1990;264:63–67. [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage JM, Kornos RL, Fung JJ, Starzl TE. The clinical trial of FK-506 as a primary and rescue immunosuppression in adult cardiac transplantation. Transplantation Proc. 1991;23:6, 3054–3057. [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith BP, Bando K, Hardesty RL, et al. Prospective randomized trial of FK-506 vs. cyclosporine. Presented at American Society of Transplant Surgeons Annual Meeting; 1993. [Google Scholar]
- 5.Todo S, Tzakis A, Reyes J, et al. Clinical small bowel or small bowel plus liver transplantation under FK-506. Transplantation Proc. 1991;23(6):3093–3098. [PMC free article] [PubMed] [Google Scholar]
- 6.Ricordi C, Tzakis A, Carroll P, et al. Human Islet Allo-transplantation under FK-506. Transplantation Proc. 1991;23(6):3207. [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro R, Jordan M, Fung JJ, et al. Kidney transplantation under FK-506 immunosuppression. Transplantation Proc. 1991;23(1):920–923. [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro R, Jordan M, Scantlebury V, et al. FK-506 in clinical kidney transplantation. Transplantation Proc. 1991;23(6):3065–3067. [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro R, Jordan M, Scantlebury VP, et al. Randomized Trial of FK-506/Prednisone vs. FK-506/Azathioprine/Prednisone after renal transplantation: Preliminary Report. Transplantation Proc. 1993;25(1):669–672. [PMC free article] [PubMed] [Google Scholar]
- 10.Japanese FK-506 Study Group. Japanese study of FK-506 on kidney transplantation: Results of an early phase II study. Transplantation Proc. 1991;23(6):3071–3074. [PubMed] [Google Scholar]
- 11.Japanese FK-506 Study Group. Japanese study of FK-506 on kidney transplantation: Results of late phase II study. Transplantation Proc. 1993;25(1):649–654. [PubMed] [Google Scholar]
- 12.Neylan J, Whelchel J, Laskow D, et al. Adverse events in the comparative dose finding trial of FK-506 in primary renal transplantation. American Society of Transplant Physicians Annual Meeting; 1993. [Google Scholar]
- 13.Friedman L, Furberg CD, DeMets DL. Fundamental of Clinical Trials. 2. Mosby Year Book; 1985. pp. 54–56. [Google Scholar]
- 14.Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506: Clinical significance and comparison with cyclosporine. Am J Surg Pathol. 1993;17(1):60–68. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro R, Tzakis A, Hakala T, et al. Cadaveric renal transplantation under the American Organ Allocation System. In: Bonomini V, Scolari MP, Stefoni S, et al., editors. Biotechnology in Renal Replacement Therapy. Vol. 70. Contrib Nephrol Basel; Karger: 1989. pp. 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sollinger HW, Beierhoi MH, Belzer RO, Diethelm AG, Kauffman RS. RS-61443 - A phase I clinical trial and pilot rescue study. Transplantation. 1992;53:428–432. doi: 10.1097/00007890-199202010-00031. [DOI] [PubMed] [Google Scholar]
- 17.Makowka L, Chapman F, Cramer DV. Historical development of brequinar sodium as a new immunosuppressive drug for transplantation. Transplantation Proc. 1993;25(3):2–7. [PubMed] [Google Scholar]
- 18.Tajima A, Hata M, Ohta N, Ohtawara Y, Kazuo S, Aso Y. Bredinin treatment in clinical kidney allografting. Transplantation. 1984;38(2):116–118. doi: 10.1097/00007890-198408000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Chong AS-F, Gebel H, Finnegan A, et al. Leflunomide, a novel immunomodulatory agent: In vitro analyses of the mechanism of immunosuppression. Transplantation Proc. 1993;25(1):747–749. [PubMed] [Google Scholar]