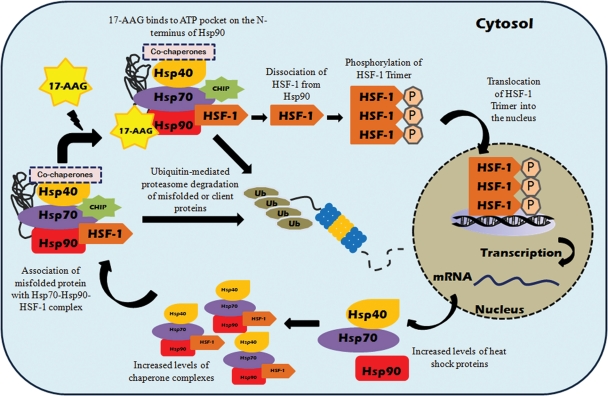
Figure 2.
17-AAG-mediated induction of heat shock response and preferential client protein degradation in mammalian cells. Under normal physiological conditions, inactive monomeric HSF-1 is localized in the cytosol and is associated with the Hsp90 multi-chaperone complex. Upon exposure to cellular stress or stress inducers, i.e. 17-AAG, which binds to the ATP pocket on the N-terminus of Hsp90, HSF-1 dissociates from the Hsp90 complex and is activated via trimerization and phosphorylation. Subsequently, activated HSF-1 translocates into the nucleus and binds to the heat shock element in the promoter region of Hsp70 and various molecular chaperone genes to simultaneously induce their expression. Binding of 17-AAG to Hsp90 can also disrupt the association of Hsp90 complex with client proteins, altering the Hsp90 complex from stabilizing form towards proteasome-targeting form, resulting in enhanced proteasome degradation of Hsp90 client proteins.