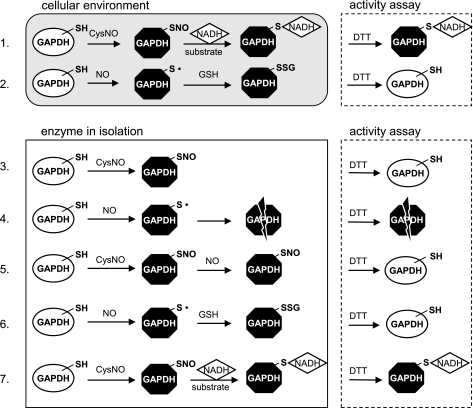
Fig. 7.
Model for a mechanism of GAPDH inhibition. 1: Inside the cell, S-nitrosated GAPDH undergoes irreversible modification, most probably via NADH attachment. 2: NO inhibits cellular GAPDH in a DTT-reversible way, and the inhibition recovers with time inside the cell, consistent with GSH repairing the cysteine in the active site (reaction between thiyl radical and GSH is simplified). 3: CysNO causes S-nitrosation of isolated GAPDH and the modification is reversible with DTT. 4: In the isolated enzyme situation, NO causes DTT-irreversible modifications of GAPDH, most probably because of secondary reactions that cannot be repaired by DTT. 5: CysNO treatment of purified GAPDH leads to S-nitrosation of the active site cysteine, thus preventing from irreversible NO-dependent inhibition of the enzyme. 6: GSH protects the isolated enzyme from irreversible NO-dependent inhibition (reaction between thiyl radical and GSH is simplified). 7: Purified S-nitrosated GAPDH undergoes irreversible inhibition under turnover conditions. Note: GAPDH monomer contains 4 cysteine residues, but for simplicity only the most reactive active site cysteine is represented. SH, protein thiol group; SNO, protein S-nitrosothiol group; S·, protein thiyl radical; SSG, glutathionylated protein.