Abstract
N-methyl-d-aspartate (NMDA) receptor-mediated currents are enhanced by phosphorylation. We have investigated effects of phosphorylation-dependent short-term plasticity of NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) on the induction of long-term depression (LTD). We confirmed in whole cell clamped CA1 pyramidal neurons that LTD is induced by pairing stimulus protocols. However, after serine-threonine phosphorylation was modified by postsynaptic introduction of a protein phosphatase-1 (PP1) inhibitor, the same pairing protocol evoked long-term potentiation (LTP). We determined effects of modification of phosphatase activity on evoked NMDA EPSCs during LTD induction protocols. During LTD induction, using a protocol pairing depolarization to –40 mV and 0.5 Hz stimulation, NMDA receptor-mediated EPSCs undergo a short-term enhancement at the start of the protocol. In neurons in which PP1 activity was inhibited, this short-term enhancement was markedly amplified. We then investigated the effect of this enhancement on Ca2+ entry during the start of the LTD induction protocol. Enhancement of NMDA receptor-mediated responses was accompanied by an amplification of induction protocol-evoked Ca2+ transients. Furthermore, this amplification required synaptic activation during the protocol, consistent with an enhancement of Ca2+ entry mediated by NMDA receptor activation. The sign of NMDA receptor-mediated long-term plasticity, whether potentiation or depression depends on the amplitude of the synaptic Ca2+ transient during induction. We conclude that short-term phosphorylation-dependent plasticity of the NMDA receptor-mediated EPSCs contributes significantly to the effect of phosphatase inhibition on the subsequent induction of LTD or LTP.
INTRODUCTION
The direction and intensity of synaptic plasticity (long-term potentiation, LTP, vs. long-term depression, LTD) is determined by the intensity of the inducing Ca2+ transient (Bear 1995; Cummings et al. 1996; Lisman 1985; Yang et al. 1999). N-methyl-d-aspartate (NMDA) receptors couple targeted postsynaptic Ca2+ influx (Alford et al. 1993) with voltage dependency (Herron et al. 1986; Hestrin et al. 1990), to initiate this Ca2+ signal. However, although plasticity of NMDA receptor-mediated synaptic responses modifies induction of further synaptic plasticity (Macdonald et al. 2007), little is known of the kinetic properties of NMDA receptor responses during initiation of LTD or LTP.
There remains uncertainty over the role of kinases and phosphatases during LTP or LTD induction. Inhibition of any of calmodulin kinase II (CaMKII) or protein kinases A, C, and G (PKA, PKC, and PKG) may prevent LTP in CA1 cells (Hussain and Carpenter 2003), but these kinases have many targets. At least 79 synaptic phosphoproteins have been identified (Collins et al. 2005), including NMDA receptors themselves (Jones and Leonard 2005; Kelso et al. 1992; Liao et al. 2001), which may, in turn, modify NMDA receptor activation of these same kinases (Raveendran et al. 2009). Because many forms of plasticity are NMDA receptor-dependent, these effects are particularly important. For example, serine threonine kinases such as PKC, and tyrosine kinases such as Src tyrosine kinase (Src), enhance NMDA currents (Liao et al. 2001; Lu et al. 1998; Macdonald et al. 2007; Yu et al. 1997). Conversely, LTD of NMDA excitatory postsynaptic currents (EPSCs) requires phosphatases (Morishita et al. 2005). Kinase and phosphatase activation generally require dendritic Ca2+ fluxes but may subsequently determine future modification of Ca2+ entry itself (Bortolotto et al. 2005; Zhang et al. 2005) and the direction and intensity of synaptic plasticity (Abraham and Bear 1996). Synaptic responses are also subject to myriad short-term influences, many of which alter postsynaptic phosphorylation. Tyrosine kinases such as Src (Kalia et al. 2004; Yu et al. 1997) and serine threonine kinases such as PKC may play important roles in the final synaptic response through the previously mentioned local modulation of NMDA currents (Liao et al. 2001).
Mechanisms underlying NMDA receptor-dependent LTD have been considered straightforward. Activation of protein phosphatase 1 (PP1) or calcineurin may de-phosphorylate AMPA receptors (Morishita et al. 2001, 2005; Mulkey et al. 1994). Indeed, PP1 may directly target AMPA receptors by acting at a motif that can, in turn, be blocked by inhibitor 1 at excitatory synapses (Morishita et al. 2005). Spinophilin, which interacts with PP1, is similarly required for LTD induction (Feng et al. 2000). Because peptides derived from these proteins prevent LTD without altering low-frequency-evoked NMDA receptor-mediated responses, these results are thought to substantiate evidence for the direct Ca2+-dependent activation of phosphatases leading to dephosphorylation of AMPA receptors and subsequently to LTD (Morishita et al. 2001). It is notable that when PP1 is used to evoke LTD, depression is only observed following NMDA receptor activation. Ca2+ influx through NMDA receptors is, thus thought to interact with PP1 to induce LTD.
But what of short-term Ca2+-dependent changes in synaptic function that might impact the induction phase of long-term synaptic plasticity? Neither inhibition of kinases (Malinow et al. 1989) nor of phosphatases (Morishita et al. 2001) alters synaptic currents during very low-frequency stimulation, but effects of phosphorylation on short-term synaptic plasticity have been neglected. Both PP1 and calcineurin modify NMDA receptor phosphorylation states (Raveendran et al. 2009) and NMDA receptor phosphorylation fluctuates depending on stimulation paradigms (Tong et al. 1995). We have investigated the effect of PP1 inhibition on modulation of NMDA EPSCs during LTD induction. Inhibition of PP1 prevents and even reverses LTD to evoke LTP, and we demonstrate that this inhibition potentiates NMDA currents and consequent dendritic Ca2+ entry during LTD induction. We conclude that short-term plasticity during LTD induction will contribute to the observed effects of inhibition of phosphatases on LTD and may provide a simpler explanation for the apparent specificity of peptide phosphatase inhibitors to LTD than has previously been proposed (Morishita et al. 2001).
METHODS
Slice preparation
Acute transverse hippocampal slices (300 μm) were prepared from 18 to 21 day Sprague-Dawley rats decapitated under deep halothane anesthesia. The hippocampus was placed in cold artificial cerebrospinal fluid (ACSF, in mM; 122 NaCl, 30 NaHCO3, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 10 d-glucose, and 2 CaCl2) and sectioned with a tissue slicer. Slices were immersed in ACSF at 32–35°C for 30–45 min then allowed to reach room temperature. For recording, slices were transferred to a recording chamber at room temperature (∼21°C). These procedures conformed to institutional guidelines as determined by the University of Illinois at Chicago Animal Care Committee from whom an appropriate protocol has been approved.
Electrophysiology
Whole cell recordings were from CA1 pyramidal cells. Patch pipettes (4–5 MΩ) were filled with solution containing (in mM): 146 cesium-methane sulfonate, 10 HEPES, 0.1 EGTA, 2 MgCl2, 4 Mg-ATP, 2 Na-GTP, and 5 QX-314 (pH 7.3). Cells were held at –60 mV and stimulated at 30 s intervals for low-frequency measurements of EPSC amplitudes. In graphs showing long-term effects, each two sequential responses were pooled for clarity. Isolated NMDA currents were recorded using bath applied 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 5 μM) and picrotoxin (50 μM) or bicuculline methiodide (5 μM). LTD was induced with a pairing stimulus of 0.5 Hz coupled with a postsynaptic depolarization to –40 mV for 10 min. LTD induction protocols were applied within 10 min of obtaining whole cell access. Protein phosphatase inhibitor 2 (100 nM) was obtained from Calbiochem, San Diego, CA. Amino acid receptor antagonists were obtained from Tocris Bioscience, Ellisville, MO.
Imaging
For Ca2+ imaging experiments Oregon Green 488 bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA) 1 (50 μM) was substituted in the patch pipette for EGTA, otherwise recording conditions were similar to those described for electrophysiology. Slices were imaged under a ×40, 0.85 NA 3.2 mm working distance lens (Olympus). Imaging was performed with a customized Biorad 600 confocal microscope. Excitation using laser lines at 488 and 568 nm simultaneously detected through a band-pass emission filter (510–560 nm) and a long-pass filter (570 nm). Signal was amplified with low noise current to voltage converters (Stanford Instruments) and sampled at 5 MHz, 10 bits through a National Instruments A-D board using custom software (http://alford.bios.uic.edu). With the confocal aperture fully open, this arrangement allowed simultaneous imaging of most of the recorded pyramidal neuron filled with Oregon Green 488 BAPTA 1. The dendritic tree was filled to acceptable resolution within 5 min of obtaining whole cell access. The first pairing stimulus was always applied within 5 min of initial recording. During the pairing, stimulation image sequences were recorded at 2 Hz.
Statistics
Student's t-test was used to assess significance. For LTP and LTD experiments, the mean of 10 final data points following pairing was compared with the mean of the baseline points to determine significance. Results were shown as significant if the P value was <0.05. Errors are expressed as SE.
RESULTS
Induction of long-term synaptic plasticity
We first confirmed protocols that initiate reliable long-term plasticity. We wished to apply agents to modify phosphorylation solely in the recorded postsynaptic neuron and thus needed to record long- and short-term synaptic modifications while introducing blocking agents through a whole cell pipette. Pairing protocols, in which selective depolarization of the whole cell clamped neuron isolates synaptic modification to just the recorded neuron, are particularly useful for these experiments. The disadvantage of using this protocol is that induction of long-term changes in the efficacy of synaptic transmission mediated by NMDA receptors is subject to rundown (Malinow and Tsien 1990). Thus the experimental protocol requires that pairing be delivered within ∼10 min of obtaining whole cell access to prevent loss of an, as yet, unknown component by dialysis through the whole cell pipette.
After obtaining whole cell access to a CA1 pyramidal neuron, the Schaffer collateral commissural pathway (SCCP) was stimulated at 30 s intervals to record control EPSCs. A pairing protocol (whole cell voltage clamp depolarization to −40 mV; SCCP stimulation, 0.5 Hz at test intensity; 10 min) was then applied, either within 10 min of obtaining whole cell access, or after 20 min. If pairing was applied after 20 min, the response showed little or no change over control amplitudes (the response 30 min after pairing was 102 ± 18% of the amplitude prior to pairing; 5 cells in 5 preparations, not shown).
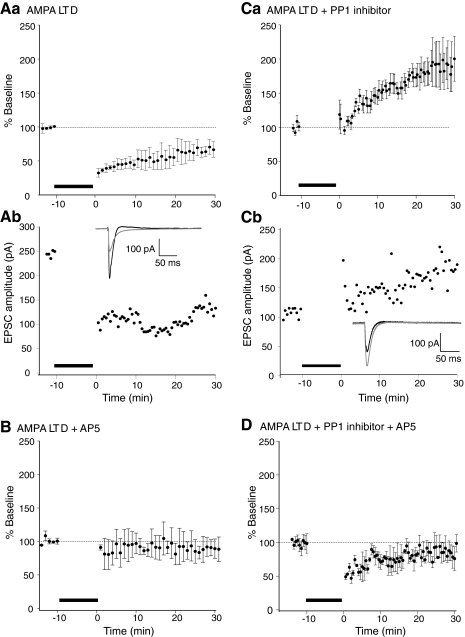
In contrast, to ensure LTD was induced, control EPSCs were recorded for ≤10 min following whole cell access and prior to application of the same pairing induction protocol. LTD was reliably induced by the application of the same pairing protocol. LTD of the subsequent EPSCs was recorded for 30 min in all cells tested (Fig. 1A; depressed to 67 ± 10% of control 30 min post pairing, n = 4 in 4 preparations). This result is very similar to those found by previous studies (Morishita et al. 2001) but provides a baseline measurement to ensure that this protocol reliably induces LTD. This pairing evoked LTD is NMDA receptor-dependent. A further four cells were recorded under the same conditions, but the NMDA receptor antagonist d-2-amino-5-phosphonopentanoic acid (d-AP5, 50 μM) was applied to the superfusate. The same pairing induction protocol now failed to evoke any change in the EPSC amplitude (Fig. 1B) either immediately or 30 min after the application of the induction protocol (EPSC amplitude immediately after the induction protocol was 92 ± 8% and after 30 min was 91 ± 14% of preinduction amplitude).
Fig. 1.
Postsynaptic protein phosphatase-1 (PP1) inhibition results in an AMPA long-term potentiation (LTP) in response to long-term depression (LTD)-inducing stimulation. Aa: LTD induced by a 10 min pairing protocol. The holding potential was stepped to –40 mV while the Schaffer collateral commissural pathway (SCCP) was stimulated at 0.5 Hz. The bar denotes the induction period. Pooled data from 5 neurons are shown. Following LTD-inducing stimulus, excitatory postsynaptic current (EPSC) amplitudes were significantly depressed (to 67 ± 10% of baseline 30 min after induction). Ab: example recording from 1 cell. Inset: traces are shown from before LTD induction and after 30 min postinduction. B: this LTD is N-methyl-d-aspartate (NMDA) receptor-dependent. The experiments were repeated in d-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μM). No depression was evoked by induction protocols identical to Aa. Pooled data from 5 neurons are shown. Ca: protein phosphatase inhibitor 2 was included in the patch pipette. Following the LTD-inducing stimulus, postsynaptic PP1 inhibition results in an AMPA LTP in response to an LTD-inducing stimulus. Currents significantly potentiate to 183 ± 28% of baseline. Pooled data from 5 neurons. Cb: an example recording and inset traces (black, before; gray, 30 min after pairing) are shown. D: this LTD protocol induced potentiation is NMDA receptor-dependent. The experiments were repeated in d-AP5 (50 μM). A short-term depression was evoked by induction protocols identical to Aa and Ca. Pooled data from 4 neurons is shown.
Inhibition of phosphatases has been demonstrated previously to prevent induction of LTD (Morishita et al. 2001; Mulkey et al. 1994). Therefore we confirmed that inhibition of phosphatases can modify LTD induction under our experimental conditions. We used a selective protein phosphatase 1 peptide inhibitor (inhibitor 2; 100 nM) applied in the whole cell pipette. As for control LTD experiments, in a further five neurons (5 preparations), CA1 pyramidal neurons were held in whole cell voltage clamp, and baseline responses evoked at 30 s intervals at a holding potential of –60 mV. Inhibitor 2 was included in the patch pipette, and we, again, attempted to induce LTD using an identical pairing protocol (whole cell voltage clamp depolarization to −40 mV; SCCP stimulation, 0.5 Hz at test intensity; 10 min). Under these conditions, all of the recorded cells exhibited a late onset LTP of the AMPA receptor-mediated EPSC following what in control conditions was an LTD-inducing stimulus (Fig. 1C, after 30 min EPSC was enhanced to 183 ± 28% of prestimulus amplitude, n = 5, significantly greater than responses recorded 30 min after control LTD induction, P < 0.01 and significantly greater than preinduction responses in these experiments, P < 0.05). Similarly to LTD induced under control conditions, this potentiation was also NMDA receptor-dependent. In 5 cells in which inhibitor 2 (100 nM) was included in the patch pipette, NMDA receptor responses were blocked by addition of d-AP5 (50 μM) to the superfusate. The same LTD pairing protocol was once more applied. In these cells, a transient depression was evoked, but the responses returned to preinduction amplitudes after 30 min of recording (Fig. 1D; 30 min postinduction the response returned to 98 ± 13% of the preinduction amplitude). We conclude that inhibition of phosphatase activity can reverse NMDA receptor-dependent LTD induction, causing a later sustained NMDA receptor-dependent potentiation of the response.
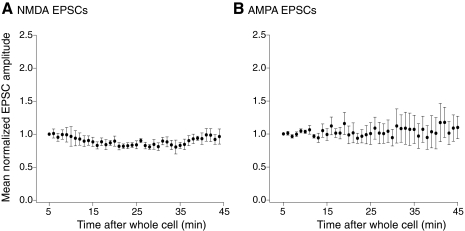
The success of LTD induction was not only dependent on the pairing protocol, but it was also necessary that the protocol occurred within 10 min of obtaining whole cell access to the recorded neuron. Consequently, only a limited preinduction recording period could be obtained prior to induction of long-term synaptic plasticity. Previous work has demonstrated that phosphatase inhibition in the absence of induction protocols, did not alter the evoked synaptic response (Morishita et al. 2001). We confirmed this under these recording conditions both for NMDA receptor-mediated and AMPA receptor-mediated synaptic responses. In five neurons, the tissue was superfused with the AMPA receptor antagonist CNQX (5 μM) and the GABAA receptor antagonist, bicuculline (5 μM), and evoked responses obtained at 30 s intervals in neurons recorded under whole cell conditions with inhibitor 2 (100 nM) included in the whole cell pipette. The resultant NMDA receptor-mediated EPSCs remained similar in amplitude for 40 min of recording (Fig. 2A; after 40 min of recording mean NMDA EPSC amplitude was 96 ± 11% of the initial recorded amplitude, not significantly changed over the course of the experiment). In a further four neurons EPSCs were recorded with inhibitor 2 (100 nM) with no antagonists in the superfusate. Again the resultant evoked AMPA receptor-mediated EPSCs remained constant in amplitude over 40 min of recording (Fig. 2B; after 40 min of recording mean AMPA EPSC amplitude was 110 ± 18% of the initial recorded amplitude, not significantly changed over the experiment). It is apparent from these experiments and earlier reports, (Malenka and Bear 2004; Morishita et al. 2001, 2005; Mulkey et al. 1993) that phosphatase inhibition does not modify responses evoked at low stimulation frequencies, either NMDA or AMPA receptor-mediated. However, serine-threonine phosphorylation can markedly alter NMDA receptor currents (Jones and Leonard 2005; Kelso et al. 1992; Liao et al. 2001). Thus we sought to investigate effects of phosphatase inhibition on NMDA receptor-mediated responses during the induction protocol itself.
Fig. 2.
Both AMPA and NMDA receptor-mediated EPSCs remain stable in amplitude in neurons recorded with pipettes containing inhibitor 2. A: mean NMDA receptor-mediated EPSC amplitudes (n = 4) normalized to the amplitude of the 1st minute of recording after whole cell access with pipettes containing inhibitor 2. The superfusate contained 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 5 μM) and bicuculline (5 μM). B: mean AMPA receptor-mediated EPSC amplitudes (n = 5) normalized as in the preceding text recorded with pipettes containing inhibitor 2 (100 nM).
Short-term phosphorylation-dependent plasticity of the NMDA EPSC
Because phosphatases play a role in LTD induction, it has been proposed that LTD induction requires dephosphorylation of either the AMPA receptor or of elements responsible for receptor insertion into the dendritic spine membrane (Kessels and Malinow 2009). However, the effect of phosphatase inhibitors on the robustness of the underlying induction protocol itself has not been examined carefully following phosphatase inhibition. Phosphorylation can substantially alter NMDA receptor currents both by direct (Kelso et al. 1992; Liao et al. 2001) and indirect (Lan et al. 2001; Lin et al. 2006) effects on the receptor. Thus we hypothesize that phosphatases may also play a metaplastic role in the induction of LTD by altering NMDA receptor responses in the short-term.
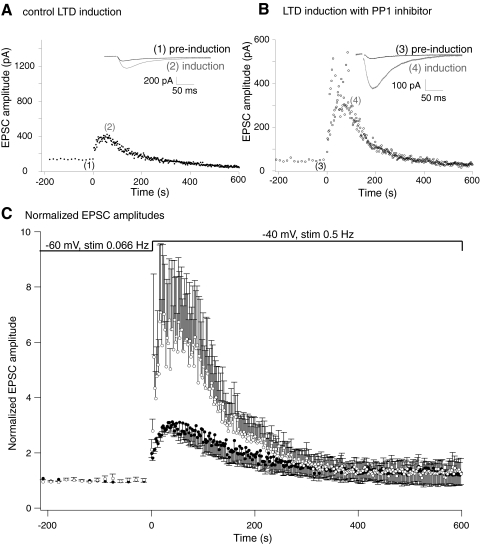
Alterations in NMDA receptor function during the induction protocol might alter induction of long-term plasticity. We quantified pharmacologically isolated NMDA receptor-mediated synaptic responses during LTD induction protocols identical to those used to evoke AMPA receptor LTD in control conditions and after intracellular application of the inhibitor 2 peptide. Cells were recorded in whole cell conditions in CNQX (5 μM) and picrotoxin (PTX; 50 μM) to block AMPA receptor and GABAA receptor-mediated synaptic currents. GABAB receptor-mediated responses were blocked by inclusion of Cs+ as the major cationic charge carrier in the pipette, to prevent K+ channel activation. This isolated the NMDA receptor-mediated EPSC. Stable preinduction responses were recorded at 15 s intervals at –60 mV (Fig. 3A). In control neurons, in which no inhibitor 2 was included in the pipette, an LTD induction protocol was then applied, in which the neurons were stepped to –40 mV and stimulated at 2 s intervals for 300 stimuli. As expected for an NMDA receptor-mediated EPSC, the first response recorded at –40 mV was substantially enhanced (to 2.0 ± 0.2 times preinduction amplitude) due to loss of voltage-dependent Mg2+ block of the NMDA receptor-mediated synaptic response. However, later responses exhibited a marked short-term potentiation followed by a later depression as the induction protocol proceeded (EPSCs rose to a peak of 3.1 ± 0.2 times the preinduction amplitude after a mean of 15 stimuli following the step to –40 mV, n = 5, 5 preparations, or 1.5 ± 0.2 times the 1st response recorded as the cell was stepped to –40 mV).
Fig. 3.
Short-term plasticity of NMDA receptor-mediated EPSCs during LTD induction. Pharmacologically isolated NMDA receptor-mediated EPSCs were recorded before and during the LTD induction protocol. A: graph of NMDA EPSC amplitude. Responses were recorded at 15 s intervals at –60 mV prior to the induction protocol. The membrane potential was then stepped to –40 mV and responses recorded at 2 s intervals. Inset: traces are example EPSCs from the preinduction recording (1, at –60 mV) and at the peak of the EPSC amplitude during the induction protocol (2, at –40 mV). B: neuron recorded under the same conditions as (A) but the PP1 inhibitor peptide was included in the whole cell patch solution. Note that in A and B, the initial amplitude of the EPSC was not the same (this varied from 50 to 120 pA across all recordings). Therefore the graph y axes are adjusted to give approximately comparative initial scaling. C: normalized recordings from 5 cells under control conditions (●) and 5 cells in which the PP1 inhibitor peptide was included in the whole cell patch solution (○). EPSC amplitudes were normalized to preinduction amplitudes during stimulation at –60 mV. Inhibition of PP1 significantly enhances the short-term potentiation of the NMDA receptor-mediated EPSC.
A similar protocol was applied to cells recorded with inhibitor 2 (100 nM) in the whole cell solution but that were otherwise treated similarly to control conditions. Similar to control responses, and to earlier reports in which phosphatase inhibitors were applied to CA1 neurons (Morishita et al. 2001), the NMDA receptor-mediated responses recorded after obtaining whole cell access were stable (Fig. 2A), indicating that the inhibitor neither enhanced nor depressed the NMDA receptor-mediated EPSC recorded at –60 mV and stimulated at 15 s intervals. A similar result for a shorter period before the induction protocol is seen in these data (Fig. 3B). As in the preceding text, an LTD induction protocol was then applied. During the LTD induction protocol the first EPSC recorded at –40 mV was enhanced similarly to those recorded in control neurons (to 2.1 ± 0.4 times the preinduction amplitude; not significantly different from control response, n = 5). This indicates that at this voltage range inclusion of inhibitor 2 did not alter the voltage dependence of the NMDA receptor-mediated response. However, subsequent EPSCs demonstrated a markedly greater short-term enhancement (to 7.1 ± 2.5 times the preinduction amplitude, n = 5; Fig. 3, B and C, or 3.3 ± 0.8 times the 1st response recorded as the cell was stepped to –40 mV). This peak enhancement was significantly greater than the control enhancement (P < 0.05, pooled data Fig. 3C). Thus modifying phosphatase activity in the synapse allows a substantial modification of NMDA receptor EPSC amplitude during induction, but not at low frequency stimulation at –60 mV when Ca2+ entry through NMDA receptors is minimal.
Access of the inhibitor peptide to the synapse
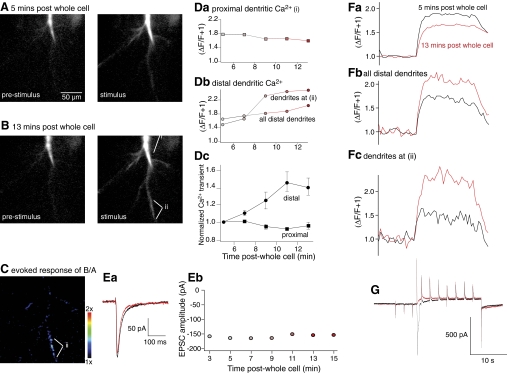
To induce LTD reliably, it was necessary to apply the induction pairing protocol within 10 min of obtaining whole cell access. It is likely that the peptide did indeed access postsynaptic terminals within this time course because its application significantly altered LTD induction (Fig. 1) as well as short-term plasticity of the NMDA component of the synaptic response (Fig. 3). However, we next wished to evaluate the efficacy of PP1 inhibition on Ca2+ entry mediated by the pairing protocol to determine whether modifications of NMDA receptor-mediated responses during the induction protocol also alter evoked Ca2+ entry. To do so, it was necessary to confirm comparative diffusion rates of small molecule Ca2+-sensitive dyes (MW ∼500) and polypeptide inhibitors (MW ∼31,000) within the 10 min window used for these protocols.
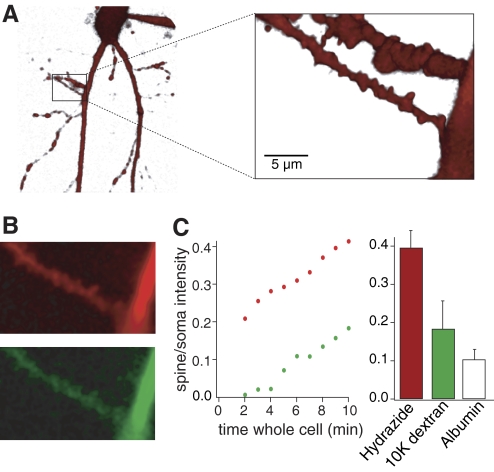
For inhibitor 2 to be present at the locus of induction of a long-term plastic change at these synapses, it must diffuse from soma to synapse within the previously defined 10 min window of recording prior to pairing. We determined that diffusion from a whole cell pipette was feasible in this time by imaging diffusion of fluorescent dyes of differing molecular weights from the pipette to the dendrites. Two dyes were included in the patch solution Alexa 594 hydrazine (MW 520) and Oregon Green 488 bound to a 3000 MW dextran amine (total molecular weight of ∼3,500). After whole cell access, the cell was imaged confocally (excitation at 488 and 568 nm). The soma was immediately labeled with both dyes on obtaining whole cell access. Diffusion into the dendrites took longer with a significant delay loading the dextran-conjugated dye. However, within 10 min both dyes were visible in proximal dendritic spines (Fig. 4, A and B). The mean spine fluorescence of the 3000 MW dextran recorded in the box in Fig. 4A was 20% of the somatic fluorescence (C). Thus dye diffusion rates are substantially altered by molecular weight of the dye complex. To provide an estimate of inhibitor 2 diffusion, we also determined similar diffusion rates for a marker with a larger molecular weight than the inhibitor. The experiments were repeated with a combination of low molecular weight Alexa 594 hydrazine, and albumin (MW 66000) labeled with Alexa fluor 488. In all six cells tested, the low molecular weight dye reached 40 ± 4% of the somatic concentration within 10 min of obtaining whole cell access, the 3,000 MW dextran reached 18 ± 7% (n = 3) and albumin reached 10 ± 3% (Fig. 4C). These numbers likely underestimate the relative concentration of the dye between spine and soma because the spine depth was similar to the z-plane resolution of the confocal microscope (∼0.5 μm), a geometry that will reduce the spine fluorescence signal. Thus peptides with molecular weights of ∼31,000 (e.g., inhibitor 2) will diffuse to the target dendritic spines within the 10 min recording window prior to application of pairing stimuli to evoke LTD. Note that we applied 100 nM inhibitor 2 (Kd of ∼2 nM) through the patch pipette. This approach, which would provide very little if any inhibitor at times <5 min whole cell, will nevertheless provide effective concentrations of inhibitor at the dendritic spine after 10 min of whole cell access. Thus introduction of inhibitor 2 may be predicted to begin altering spine biochemistry after ∼5 min of whole cell recording. The smaller molecular weight dye (Alexa hydrazine) was visible within dendritic spines within 2 min of obtaining whole cell access. Thus by using a low molecular weight Ca2+ sensitive dye that will reach the dendrites earlier in combination with a peptide phosphatase inhibitor (inhibitor 2) it is possible to record the effect of inhibitor 2 on Ca2+ signals as the inhibitor diffuses into distal dendrites and spines.
Fig. 4.
Diffusion rates from the whole cell pipette to CA1 pyramidal neuron dendritic spines. A: live CA1 pyramidal cell visualized with Alexa 594 hydrazine (MW 520) introduced into the cell through a patch pipette. Box magnified to reveal dendrites and dendritic spines. B: single optical sections imaging Alexa 594 hydrazine and Oregon Green 488 dextran 3000. Top: dendrite (red) with Alexa 594 10 min following whole cell access. Bottom: same dendrite (green) with Oregon Green 488, (3000 MW dextran) again at 10 min after whole cell access. C: graph showing fluorescence rise of the 2 dyes at spines recorded in B plotted against time after whole cell access. Data are normalized (spine intensity/soma intensity). Red, Alexa 594; green, Oregon Green. Histograms show the relative intensity of Alexa 594 hydrazide (red), Oregon Green dextran (green) and fluorescently labeled albumin (MW 66,000; white) introduced in a further 3 neurons, between the soma and dendritic spines, 10 min after obtaining whole cell access.
PP1 inhibitor peptide enhances Ca2+ entry during LTD protocols
The transient dendritic Ca2+ concentration during induction of long-term plasticity is thought to be particularly important for the sign of any evoked long-term change in synaptic response amplitude—whether the outcome is potentiation or depression. High Ca2+ concentrations are believed to evoke LTP, lower concentrations, although to values greater than rest, and sustained for minutes, evoke LTD (Abraham and Bear 1996). We have demonstrated that inhibiting phosphatase activity causes a short-term enhancement of the NMDA receptor-mediated responses during protocols that in control conditions cause LTD induction but after inhibitor 2 application instead induce LTP. We therefore sought to determine whether postsynaptic treatment with inhibitor 2 alters Ca2+ entry during LTD induction protocols.
Pyramidal neurons were held under whole cell voltage clamp with pipettes containing a Ca2+-sensitive dye (Oregon Green 488 BAPTA 1; 50 μM) and the phosphatase inhibitor (inhibitor 2, 100 nM). Immediately after obtaining whole cell access, synaptic currents were evoked by stimulating the SCCP. Ca2+ dye fluorescence was visible at the somata of these neurons but was not sufficiently bright to image beyond the primary dendrites. Over the next 5 min the resting Ca2+ dye fluorescence gradually increased similarly to images of low molecular weight dyes (Fig. 4). Resting Ca2+ fluorescence was then measured over a region extending for 200 μm from the soma in the stratum radians, in a single confocal optical section (Fig. 5A). Fluorescence and whole cell current was simultaneously measured during a stimulus/voltage step protocol. This protocol was designed to mimic the start of the LTD induction protocol used in Fig. 1 during which substantial NMDA receptor-mediated synaptic enhancement was recorded (Fig. 3). However, for these imaging experiments, the protocol was terminated early so that long-term plastic changes in synaptic currents were not evoked by the protocol itself. The protocol comprised 10 stimuli to the SCCP, 3 prior to the voltage step command to monitor synaptic response amplitude, and 7 during the depolarizing pulse (Fig. 5G). This stimulus-step protocol caused a substantial rise in Ca2+-sensitive dye fluorescence throughout the recorded neuron for the duration of the depolarizing step (Fig. 5, A and F, black traces). An identical stimulus-step protocol was reapplied at 2 min intervals, and the amplitude of the resultant fluorescence transient was recorded and plotted as (ΔF/F + 1) over time. For the duration of these recordings, the individual synaptic responses (EPSCs) remained unchanged in amplitude (Fig. 5E).
Fig. 5.
Postsynaptic application of inhibitor 2 caused an enhancement of LTD stimulus protocol evoked Ca2+ entry. Neurons were held under whole cell voltage clamp with pipettes containing the Ca2+ sensitive dye, Oregon Green bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA) 1 and Inhibitor 2 (100 nm). A: single optical section confocal image of this dye obtained 5 min after obtaining whole cell access. Left: image is at rest immediately prior to the stimulus. Right: image is from the last image frame obtained during application of a truncated LTD induction protocol in which synaptic stimulation (0.5 Hz) was applied during a voltage step lasting for 20 s (electrophysiological response in G). B: images obtained as for A in the same neuron but after 13 min of whole cell access. C: after subtracting background data and subthreshold masking of data (see methods), stimulus evoked images in B was divided by that in A. This emphasizes that stimulus evoked fluorescence transients were nonuniformly enhanced in the 8 min between recordings in A and B. D: peak amplitude of stimulus evoked fluorescence transients measured in the dendrites for time points from 5 to 13 min after obtaining whole cell access. Da: measurements from the proximal dendritic region i in B. Db: measurements were from the region identified as ii in B and C where a substantial increase in fluorescence was seen and from all dendrites excluding the primary dendrite i in B. Dc: normalized change in amplitude of evoked fluorescence transients over time after whole cell access in all cells in which inhibitor 2 was included in the whole cell pipette. E: amplitude of the stimulus evoked synaptic responses recorded immediately before the step protocol from 3 to 15 min after obtaining whole cell access. Ea: traces obtained from cell in A–D from 3 and 15 min post whole cell. Eb: peak amplitudes of EPSCs recorded throughout this period. F: traces showing fluorescence transients comparing responses from stimuli 5 min post whole cell access (black) to 13 min after obtaining access (red). Fa: from proximal dendrites i in B. Fb: all distal dendrites. Fc: distal dendrites labeled ii in B. The response was markedly enhanced over time in the distal dendrites and depress in the proximal dendrites. G: current traces recorded at 5 min (black) and 13 min (red) after obtaining whole cell access. These were recorded simultaneously to the fluorescence transients shown in F with the same time base.
Fluorescence transients were recorded from the primary dendrites (defined as the dendrite extending directly from the soma prior to branching), as well as from all dendrites secondary to this region (defined as distal dendrites). The amplitude of the Ca2+ transient obtained in primary dendrites demonstrated a reduction in amplitude over time (Fig. 5, Da and Fa), as did the amplitude of early inward current recorded through the patch pipette (G). This likely reflects a run-down of voltage gated Ca2+ current and is an inevitable feature of whole cell recording under these conditions. In contrast, in these neurons in which inhibitor 2 was included in the whole cell recording pipette, the amplitude of the secondary dendritic fluorescence transient grew larger over time (Fig. 5, Db and Fb). These data were quantified for all nine neurons examined to show a significant increase in fluorescence in the secondary dendrites during the recording period (Fig. 5Dc; n = 9, P < 0.01). To isolate the source of this increased fluorescence, hotspots were identified by dividing the signal (ΔF/F) derived at 13 min post whole cell access by the signal obtained initially at 5 min (Fig. 5C). A region identified this way in (Fig. 5C, labeled as ii) was analyzed separately. This region showed a substantial increase in Ca2+ transient amplitude over time (Fig. 5, Db and Fc). Thus under conditions in which an LTD inducing protocol would have caused a late onset LTP, when PP1 is present in the patch pipette, the amplitude of the induction protocol-induced dendritic Ca2+ transient is substantially and significantly enhanced from its initial condition before inhibitor 2 had sufficient time to diffuse throughout the neuron. We therefore sought to determine the effect of control recording on Ca2+ transient amplitudes over the same time course.
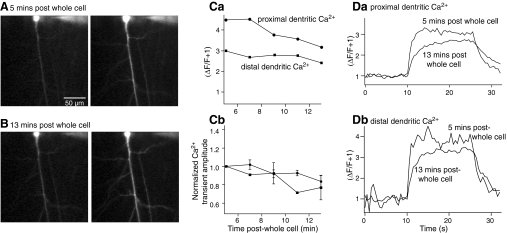
We used the same experimental protocol except the phosphatase inhibitor was omitted from the whole cell patch solution. At 5 min post whole cell access, a step-stimulus protocol was applied to the recorded pyramidal neuron and the Ca2+ transient recorded (Fig. 6A). Proximal and distal dentritic Ca2+ transients were recorded as before (Fig. 6C). These responses were also recorded at 2 min intervals. The responses in both proximal and distal dendrites both declined in amplitude from 5 to 13 min post whole cell access (Fig. 6, B and C). Similar experiments were performed in seven recorded neurons. The evoked Ca2+ transients demonstrated a significant reduction in amplitude in both proximal and distal dendrites (Fig. 6Cb; P < 0.05). Thus introduction of the phosphatase inhibitor into the whole cell pipette caused a significant increase in the amplitude of the Ca2+ transient recorded in the distal dendritic tree in response to a stimulus-step protocol that mimics the start of an LTD induction protocol. This represents a similar condition in which inhibitor 2 also causes a substantial short-term enhancement of the NMDA component of the synaptic response.
Fig. 6.
Ca2+ transients in control neurons do not show recording time-dependent enhancement. Neurons were held under whole cell voltage clamp with pipettes containing the Ca2+ sensitive dye, Oregon Green BAPTA 1. A: single optical section confocal image of this dye obtained 5 min after obtaining whole cell access. Left: image is at rest immediately prior to application of the stimulus. Right: image is from the last image frame obtained during application of a truncated LTD induction protocol in which synaptic stimulation (0.5 Hz) was applied during a voltage step protocol lasting for 20 s. B: images obtained as for A in the same neuron but after 13 min of whole cell access. C: peak amplitude of stimulus evoked fluorescence transients measured in the dendrites for time points from 5 to 13 min after obtaining whole cell access. Ca: measurements from the proximal and distal dendritic regions. Proximal dendrite was defined as the primary dendrite from the soma, distal dendrites as all others. Cb: normalized change in amplitude of evoked fluorescence transients over time after whole cell access in all control. D: traces showing fluorescence transients comparing responses from stimuli 5 min post whole cell access to 13 min after obtaining access. Da: from proximal dendrites. Db: all distal dendrites.
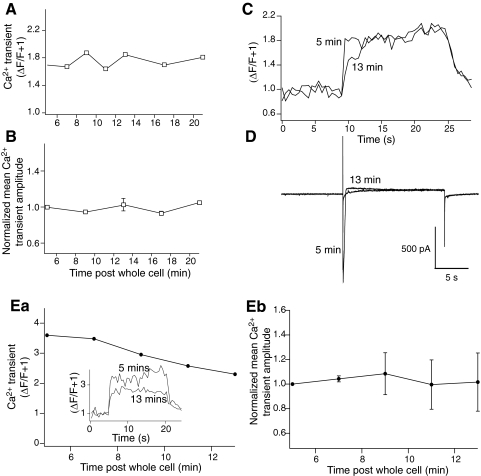
We therefore determined whether stimulation of the SCCP was necessary to record this Ca2+ transient enhancement when inhibitor 2 was included in the patch pipette. Neurons were recorded under whole cell conditions as before and with inhibitor 2 (100 nM) in the whole cell pipette. After 5 min of maintained whole cell access, a step protocol was applied to the neuron but with no accompanying stimulation of the SCCP. This protocol caused a rise in fluorescence throughout the neuron (Fig. 7A). This step protocol was applied at 2 min intervals as per the step-stimulus protocols already described. Ca2+ transients were recorded and again calculated as (ΔF/F + 1) from the proximal and distal dendrites until 21 min post whole cell access (Fig. 7, B and C). Under these conditions, with no stimulus applied to the SCCP, the amplitude of the step-evoked Ca2+ transient declined slightly during the course of the recording. Taking these data in combination with those from Figs. 3–6, we may conclude that inclusion of inhibitor 2 in the patch pipette significantly enhanced the amplitude of the Ca2+ transient mediated by synaptic input to the neurons during the early stages of an LTD induction protocol.
Fig. 7.
Inhibitor 2 did not cause a time-dependent modification of Ca2+ transients recorded following step protocols with either no synaptic or no NMDA component. Neurons were recorded as for Figs. 5 and 6 but no stimulation of synaptic inputs to the CA1 region was applied. A: peak amplitude of stimulus evoked fluorescence transients obtained during a 20 s voltage step to –40 mV, measured in the distal dendrites for time points from 5 to 21 min after obtaining whole cell access. B: normalized peak amplitude of evoked fluorescence transients over time after whole cell access in all controls for all neurons recorded. C: traces from cell in A showing fluorescence transients comparing responses from stimuli 5 min post whole cell access to 13 min after obtaining access from distal dendrites. D: current traces recorded at 5 and 13 min after obtaining whole cell access. These were recorded simultaneously to the fluorescence transients shown in C with the same time bases. Ea: peak amplitude of stimulus evoked fluorescence transients recorded in d-AP5 (50 μM) obtained during a 20 s voltage step to –40 mV while stimulating at 2 s intervals (as in Fig. 5) measured in the distal dendrites for time points from 5 to 13 min after obtaining whole cell access. (Inset: examples of Ca2+ transients at 5 and 13 min after obtaining whole cell access.) Eb: normalized peak amplitude of evoked fluorescence transients over time after whole cell access all neurons recorded under conditions in Ea.
It is possible that the inhibitor 2 peptide mediated enhancement in dendritic Ca2+ transients is caused by AMPA receptor components of the synaptic response transiently depolarizing the dendritic field because the space clamp properties of CA1 pyramidal neurons are far from ideal. To address this possibility, further control experiments were performed in which inhibitor 2 peptide (100 nM) was included in the patch pipette solution but NMDA receptor-mediated responses were blocked by d-AP5 (50 μM). The neurons were recorded from as before, and distal dendritic Ca2+ transients were obtained in response to step depolarization and 10 stimuli applied to the SCCP to evoke only non-NMDA receptor-mediated responses. The amplitude of the Ca2+ transient was recorded between 5 and 13 min of obtaining whole cell access (Fig. 7E). Over this period, the Ca2+ transient showed no significant change in amplitude (mean normalized transient amplitude after 13 min was 101 ± 23% of the response amplitude after 5 min of recording.
To induce LTD (Fig. 1), the induction protocol was applied within 10 min of obtaining whole cell access. At this time point, without manipulation of postsynaptic phosphatase activity, the Ca2+ signal in the distal dendrites during the step-stimulus protocol was slightly smaller than the earliest possible recorded control response. In contrast in responses recorded when inhibitor 2 was included in the recording pipette and in which NMDA receptor-mediated responses were evoked, the Ca2+ response was significantly increased compared with the earliest recorded response (P < 0.05) or compared with conditions in which only a voltage step was delivered or in which NMDA receptors were blocked with d-AP5. It is likely that this phosphatase modification of synaptically driven Ca2+ entry will have very profound effects on subsequent long-term synaptic plasticity.
DISCUSSION
Activity of kinases and phosphatases within dendritic spines modifies synaptic transmission. To a great extent, these processes drive the early stages of LTP and LTD and represent critical elements of synaptic plasticity of learning and memory. However, even if selective manipulation of specific phosphorylation events were feasible within only dendritic spines of recorded neurons, the outcome of this manipulation may have broad implications for both different stages of the maintenance of LTP or LTD and for the induction of these plastic changes.
There exists widespread consensus that NMDA receptor-mediated long-term plasticity is mediated by phosphorylation or dephosphorylation events within postsynaptic spines and dendrites (Malenka and Bear 2004; Soderling and Derkach 2000). The identity of the kinases and phosphatases involved remain less clearly defined, but evidence has implicated numerous serine-threonine kinases in LTP induction including Ca2+-calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC) (Malinow et al. 1989), cAMP-dependent protein kinase (PKA) (Frey et al. 1993; Matthies and Reymann 1993) as well as cGMP-dependent protein kinase (PKG) (Zhuo et al. 1994), casein kinase II (CKII), mitogen activated protein kinase (MAPK), and various tyrosine kinases including SRC kinase and fyn (Kalia et al. 2004; Lu et al. 1998). Similarly, various phosphatases have been implicated in the induction of LTD: in particular, protein phosphatase 1 (PP1) and protein phosphatases 2A and 2B (PP2A and PP2B or calcineurin) (Morishita and Malenka 2008; Morishita et al. 2001). It is notable that PP1 and PP2A act directly to dephosphorylate the NMDA receptor subunit NR2B at serine 1303 (Raveendran et al. 2009), a site the phosphorylation of which causes a marked potentiaition of NMDA currents (Kelso et al. 1992; Liao et al. 2001). Thus there exists a direct mechanism by which inhibition of phosphatase activity might modify NMDA receptor function. It is also widely accepted that phosphorylation or dephosphorylation events within the dendrites lead to the modification of postsynaptic AMPA receptor function, most likely by receptor insertion or removal from the postsynaptic active zone (Chung et al. 2003; Lin et al. 2009; Man et al. 2000).
LTP or LTD induction is believed to be mediated by processes that depend on stimulus strength whereby intense stimulation leading to high dendritic transient Ca2+ signals may lead to LTP, whereas lower concentration Ca2+ transients initiate LTD (Abraham and Bear 1996; Bear 1995; Lisman 1985); the outcome being determined by the relative affinities to Ca2+ of the kinases or phosphatases involved. This process may lead to a sliding threshold for the sign of long-term plasticity that is invoked, and it is particularly important to note that the strength of the NMDA receptor-mediated synaptic response is also subject to long-term changes evoked by similar stimuli to those that evoke LTP of AMPA receptor-mediated synaptic responses (Bashir et al. 1991). These phenomena include activation of similar kinases and phosphatases located in the postsynaptic dendrites (MacDonald et al. 2006). Thus LTP is subject to a metaplasticity (Abraham and Bear 1996; Bear 1995), which may force similar induction protocols to generate very different long-term effects on synaptic strength.
We have now addressed short-term implications for postsynaptic phosphatase activity and its implication for alterations in NMDA receptor activity and intensity of the Ca2+ signal during the induction protocol itself. During the induction of NMDA-dependent LTD or LTP, the amplitude of the Ca2+ signal that is derived from NMDA receptor activation is believed to be critical to the sign of the ensuing long-term change in synaptic response carried by AMPA receptors (Abraham and Bear 1996). Consequently, events or signal transduction processes that modify NMDA receptor responses will have profound effects on the induction of long-term synaptic plasticity—not just on its amplitude but also on its sign. Processes leading to enhanced Ca2+ entry during the induction protocol will favor potentiation, those that lead to a reduced Ca2+ entry will favor depression or depotentiation.
In this study, we confirm that pairing protocols can reliably evoke LTD but that phosphatase inhibition, within the recorded neuron, can reverse this phenomenon to induce a late onset LTP. We chose one phosphatase inhibitor (protein phosphatase inhibitor 2—which specifically inhibits the catalytic subunit of type 1 protein phosphatases) based on earlier work showing a role for its target and for serine-threonine dephosphorylation in LTD induction (Morishita et al. 2001). However, we do not have any expectation that this result will be specific to this class of phosphatase. The result seen here is more extreme than previous studies, which indicate a loss of LTD following PP1 inhibition (Morishita et al. 2001); however, as our data also indicate, this difference may simply reflect a balance of NMDA receptor-dependent Ca2+ entry during the induction protocol, which is more dependent on the precise properties of the induction protocol, than a specific underlying principle of signal transduction. Indeed, the protocols used to induce LTD differ between our study and that of (Morishita et al. 2001). In particular, the pairing protocol that we have used allowed us to choose stimulation rates at a sufficiently low frequency to allow ready detection and measurement of NMDA EPSCs.
Previously it has been argued that a loss of LTD following postsynaptic inhibition of phosphatases indicates a requirement for phosphatase activity in the postsynaptic terminal to modify receptor function—either removal from the postsynaptic density or modification of total conductance. There is very strong evidence for this conclusion (reviewed in (Kessels and Malinow 2009). Nevertheless the most notable observed effect of PP1 inhibition during the LTD induction protocol itself was to increase the amplitude of NMDA receptor-mediated EPSCs. Under control conditions, NMDA receptor-mediated EPSCs demonstrated a short-term enhancement at the start of the LTD induction protocol immediately following the activation of the pairing step used to depolarize the recorded neuron. Inhibition of PP1 altered this short-term plasticity, such that over the first eight stimuli of the LTD induction protocol, the NMDA component rose to ≥700% of the initial response at the start of the step.
The enhancement of the NMDA receptor-mediated component of the synaptic response following phosphatase inhibition was accompanied by a substantial increase in the amplitude of the postsynaptic Ca2+ transient recorded during the early phases of LTD inducing protocols. Furthermore, while pairing protocols used to evoke LTD cause Ca2+ entry by nonsynaptic activation of voltage operated Ca2+ channels, as well as by synaptic activation, this enhancement was only observed if synaptic stimulation was evoked during the pairing protocol. Thus while it has previously been concluded that specific actions of peptide phosphatase inhibitors follows from activity dependent recruitment of the phosphatase during induction protocols (Morishita et al. 2001), we may alternatively conclude that phosphatase activity during the LTD induction protocol serves to modify short-term plasticity of the NMDA receptor-mediated response. This drives a substantial alteration in protocol-evoked Ca2+ entry, which in turn can shape the outcome of induction events, even changing the result from LTD to LTP.
It is reasonable to conclude that the outcome of any induction protocol, be it aimed at inducing LTP or LTD, will be sensitive to both the robustness of the protocol and the state of the synapse to which it is applied. Changes in the phosphorylation state of NMDA receptors or other postsynaptic elements may alter the outcome of induction of long-term plasticity prior to any effect on, for example, AMPA receptor incorporation into the postsynaptic density. Our results confirm that phosphatase inhibition indeed leads to an occlusion of this depression but also demonstrate that a shift in the sign of plasticity of the synapse can occur. Furthermore much of this outcome may be attributed to short-term changes in Ca2+ entry during the induction protocol rather than specific effects of inhibition of phosphatases on AMPA receptors during the maintenance phase of LTD as has previously been proposed.
This very profound short-term modification of NMDA receptor-mediated synaptic responses through activation of a phosphatase during LTD induction means that separating the role of phosphatases, kinases, and downstream targets (Peineau et al. 2007) between induction and maintenance phases of LTP is not at all straightforward. These results may also help to explain why LTD, LTP, and depotentiation are apparently coupled (Delgado and O'Dell 2005). Each induction protocol may alter the sign of potentiation or depression of later protocols, depending on the long- and short-term effects on Ca2+ entry that are driven by NMDA receptor activation. Short-term plasticity during LTD induction may explain observed effects of inhibition of phosphatases on LTD. This may represent a very straightforward explanation for the apparent specificity of peptide phosphatase inhibitors to LTD (Morishita et al. 2001).
GRANTS
The work was supported by National Institute of Neurological Disorders and Stroke Grants NS-052699 to S. Alford and NS-056321 to J. P. Leonard.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank S. Kelso, A Bleckert, H. Photowala, and A Contractor for critical reading of the manuscript and/or invaluable discussions.
REFERENCES
- Abraham and Bear, 1996.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996 [DOI] [PubMed] [Google Scholar]
- Alford et al., 1993.Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol 469: 693–716, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir et al., 1991.Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature 349: 156–158, 1991 [DOI] [PubMed] [Google Scholar]
- Bear, 1995.Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron 15: 1–4, 1995 [DOI] [PubMed] [Google Scholar]
- Bortolotto et al., 2005.Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology 49, Suppl 1: 13–25, 2005 [DOI] [PubMed] [Google Scholar]
- Chung et al., 2003.Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300: 1751–1755, 2003 [DOI] [PubMed] [Google Scholar]
- Collins et al., 2005.Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem 280: 5972–5982, 2005 [DOI] [PubMed] [Google Scholar]
- Cummings et al., 1996.Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16: 825–833, 1996 [DOI] [PubMed] [Google Scholar]
- Delgado and O'Dell, 2005.Delgado JY, O'Dell TJ. Long-term potentiation persists in an occult state following mGluR-dependent depotentiation. Neuropharmacology 48: 936–948, 2005 [DOI] [PubMed] [Google Scholar]
- Feng et al., 2000.Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 97: 9287–9292, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey et al., 1993.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260: 1661–1664, 1993 [DOI] [PubMed] [Google Scholar]
- Herron et al., 1986.Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature 322: 265–268, 1986 [DOI] [PubMed] [Google Scholar]
- Hestrin et al., 1990.Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol 422: 203–225, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain and Carpenter, 2003.Hussain RJ, Carpenter DO. The effects of protein kinase C activity on synaptic transmission in two areas of rat hippocampus. Brain Res 990: 28–37, 2003 [DOI] [PubMed] [Google Scholar]
- Jones and Leonard, 2005.Jones ML, Leonard JP. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J Neurochem 92: 1431–1438, 2005 [DOI] [PubMed] [Google Scholar]
- Kalia et al., 2004.Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene 23: 8007–8016, 2004 [DOI] [PubMed] [Google Scholar]
- Kelso et al., 1992.Kelso SR, Nelson TE, Leonard JP. Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J Physiol 449: 705–718, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels and Malinow, 2009.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan et al., 2001.Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci 4: 382–390, 2001 [DOI] [PubMed] [Google Scholar]
- Liao et al., 2001.Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-d-aspartate receptor current. Mol Pharmacol 59: 960–964, 2001 [DOI] [PubMed] [Google Scholar]
- Lin et al., 2009.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 12: 879–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al., 2006.Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci USA 103: 19902–19907, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman, 1985.Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci USA 82: 3055–3057, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al., 1998.Lu YM, Roder JC, Davidow J, Salter MW. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279: 1363–1367, 1998 [DOI] [PubMed] [Google Scholar]
- MacDonald et al., 2006.MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol 18: 71–84, 2006 [DOI] [PubMed] [Google Scholar]
- Macdonald et al., 2007.Macdonald JF, Jackson MF, Beazely MA. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim Biophys Acta 1768: 941–951, 2007 [DOI] [PubMed] [Google Scholar]
- Malenka and Bear, 2004.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004 [DOI] [PubMed] [Google Scholar]
- Malinow et al., 1989.Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245: 862–866, 1989 [DOI] [PubMed] [Google Scholar]
- Malinow and Tsien, 1990.Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature 346: 177–180, 1990 [DOI] [PubMed] [Google Scholar]
- Man et al., 2000.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25: 649–662, 2000 [DOI] [PubMed] [Google Scholar]
- Matthies and Reymann, 1993.Matthies H, Reymann KG. Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. Neuroreport 4: 712–714, 1993 [DOI] [PubMed] [Google Scholar]
- Morishita et al., 2001.Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron 32: 1133–1148, 2001 [DOI] [PubMed] [Google Scholar]
- Morishita and Malenka, 2008.Morishita W, Malenka RC. Mechanisms underlying dedepression of synaptic NMDA receptors in the hippocampus. J Neurophysiol 99: 254–263, 2008 [DOI] [PubMed] [Google Scholar]
- Morishita et al., 2005.Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci 8: 1043–1050, 2005 [DOI] [PubMed] [Google Scholar]
- Mulkey et al., 1994.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486–488, 1994 [DOI] [PubMed] [Google Scholar]
- Mulkey et al., 1993.Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science 261: 1051–1055, 1993 [DOI] [PubMed] [Google Scholar]
- Peineau et al., 2007.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53: 703–717, 2007 [DOI] [PubMed] [Google Scholar]
- Raveendran et al., 2009.Raveendran R, Devi Suma Priya S, Mayadevi M, Steephan M, Santhoshkumar TR, Cheriyan J, Sanalkumar R, Pradeep KK, James J, Omkumar RV. Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J Neurochem 110: 92–105, 2009 [DOI] [PubMed] [Google Scholar]
- Soderling and Derkach, 2000.Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci 23: 75–80, 2000 [DOI] [PubMed] [Google Scholar]
- Tong et al., 1995.Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science 267: 1510–1512, 1995 [DOI] [PubMed] [Google Scholar]
- Yang et al., 1999.Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol 81: 781–787, 1999 [DOI] [PubMed] [Google Scholar]
- Yu et al., 1997.Yu XM, Askalan R, Keil GJ, 2nd, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275: 674–678, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2005.Zhang L, Kirschstein T, Sommersberg B, Merkens M, Manahan-Vaughan D, Elgersma Y, Beck H. Hippocampal synaptic metaplasticity requires inhibitory autophosphorylation of Ca2+/calmodulin-dependent kinase II. J Neurosci 25: 7697–7707, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo et al., 1994.Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature 368: 635–639, 1994 [DOI] [PubMed] [Google Scholar]