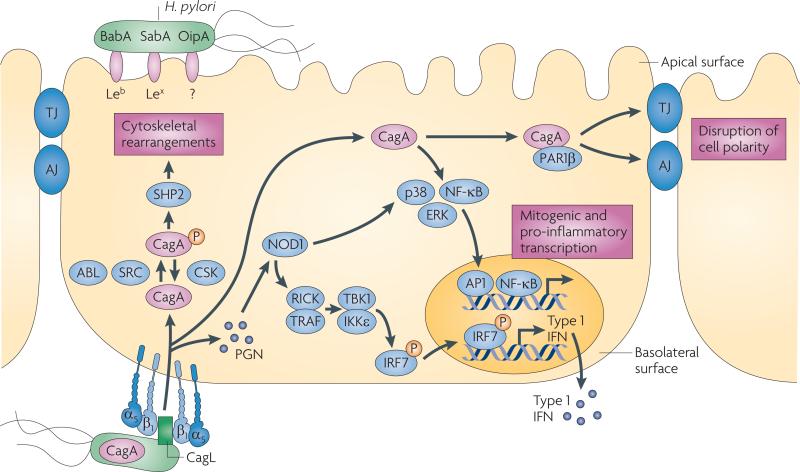
Figure 2. Interactions between pathogenic H. pylori and gastric epithelial cells.
Several adhesins such as BabA, SabA and OipA mediate binding of Helicobacter pylori to gastric epithelial cells, probably through the apical surface. H. pylori can also bind to α5β1 integrins, which are located on the basolateral surface of epithelial cells. After adherence, H. pylori can translocate effector molecules such as CagA and peptidoglycan (PGN) into the host cell. PGN is sensed by the intracellular receptor nucleotide-binding oligomerization domain-containing protein 1 (NOD1), which activates nuclear factor-κB (NF-κB), p38, ERK and IRF7 to induce the release of pro-inflammatory cytokines. Translocated CagA is rapidly phosphorylated (P) by SRC and ABL kinases, leading to cytoskeletal rearrangements. Unphosphorylated CagA can trigger several different signalling cascades, including the activation of NF-κB and the disruption of cell–cell junctions, which may contribute to the loss of epithelial barrier function. Injection of CagA seems to be dependent on basolateral integrin α5β1. AJ, adherens junction; CSK, c-src tyrosine kinase; IFN, interferon; IKKε, IκB kinase-ε; IRF7, interferon regulatory factor 7; RICK, receptor-interacting serine-threonine kinase 2; TBK1, TANK-binding kinase 1; TJ, tight junction.