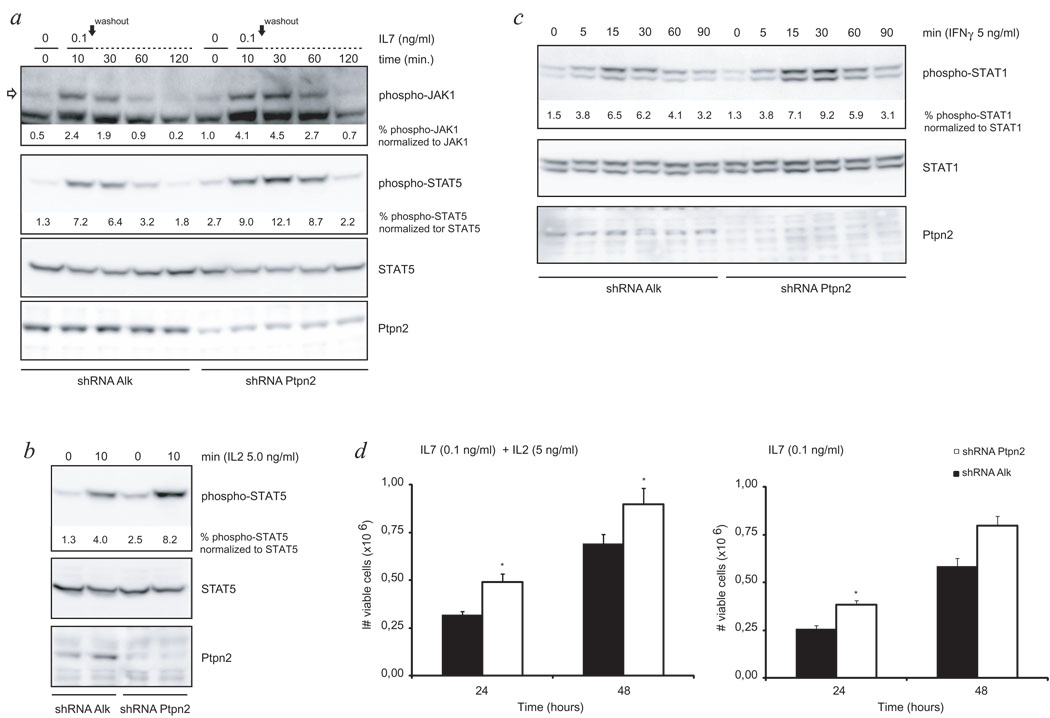
Figure 3. Mouse T-ALL cells display altered cytokine receptor signaling in correlation with Ptpn2 expression levels.
- Knockdown of Ptpn2 enhanced responsiveness of the IL7 receptor pathway to ligand stimulation as displayed by increased strength and duration of the phosphorylation status of JAK1 and downstream protein STAT5 when compared to control cells. Open arrow points at the protein band corresponding to JAK1. Black arrow indicates time point of cytokine removal. STAT5 was used as loading control.
- Western blot analysis of WCL revealed increased sensitivity of primary mouse T-ALL cells to IL2 stimulation due to decreased Ptpn2 expression as analyzed by phosphorylation level of STAT5. STAT5 is shown as loading control.
- Cytokine depleted cells were exposed to INF-γ for indicated time periods and subsequently analyzed for activation of STAT1 by western blot. Quantification of western blot experiments showed that reduction of Ptpn2 protein resulted in stronger activation of STAT1. STAT1 was assayed to ensure equal protein loading.
- Primary T-ALL cells were deprived from cytokines for 24 hours before re-stimulation with either IL7 alone or a combination of IL2 and IL7. Under both conditions, knockdown of Ptpn2 provided cells with a significant proliferative advantage in response to cytokine re-addition. Open bars: shRNA Ptpn2; closed bars: shRNA Alk (control); bars show average ± s.e.m. Error bars represent s.e.m. *p<0.05
Efficient knockdown of Ptpn2 was confirmed using anti-Ptpn2 (3E2) antibody. Differences in phosphorylation signal intensity were quantified for JAK1 and STAT proteins and corresponding values are shown below blots.