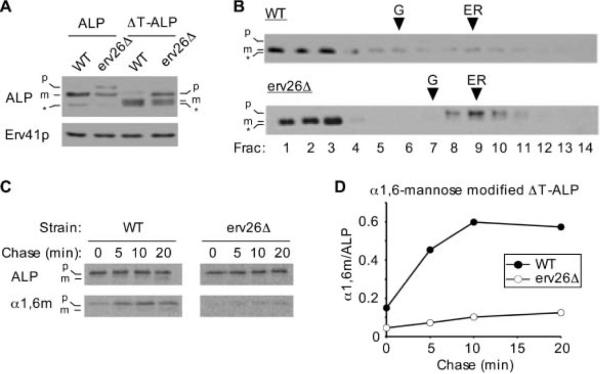
Figure 3.
The cytosolic tail of ALP is dispensable for ER export. A) Wild-type (CBY2151) and erv26Δ (CBY2240) cells expressing full-length pro-ALP (pSN92) or the tail deletion construct (pNB4) were spheroplasted by lyticase treatment. Total cell extracts were resolved on 8% polyacrylamide gels for immunoblots. Erv41p, a protein localized to ER and Golgi membranes, was used as a loading control. The pro-ALP (p), mature ALP (m) and a soluble breakdown product (*) are indicated. B) Cell extracts as in (A) were fractionated on sucrose density gradients and fractions analysed by immunoblot as described under Materials and Methods. Och1p and Sec61p served as Golgi and ER membrane markers, with peak fractions for Och1p (G) and Sec61p (ER) indicated by arrowheads. C) Pulse-chase analysis of ΔT-ALP in the wild-type and erv26Δ cells expressing the tail deletion construct (pNB4). D) Graphical representation of the results in (C) showing α1,6-mannose modified ΔT-ALP protein divided by total ΔT-ALP at each time-point.