Figure 8.
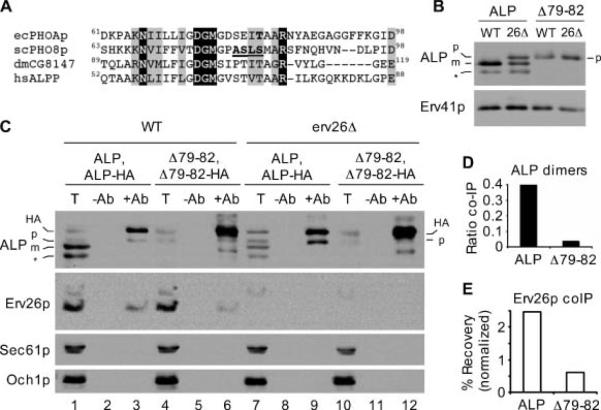
Dimerization of pro-ALP is required for efficient binding to Erv26p and ER export. A) Primary sequence alignments of Escherichia coli PhoA (GenBankTM accession BAE76164), Saccharomyces cerevisiae ALP (GenBankTM accession P11491), Drosophila melanogaster ALP (GenBankTM accession NP_649897) and human placental ALP (GenBankTM accession AAC97139). Invariant amino acids are shaded black, conserved amino acids in grey. Residues of interest are bold and underlined (see text). B) Wild-type (CBY2151) and erv26Δ (CBY2240) cells expressing full-length pro-ALP (pFL8) or the Δ79-82ALP construct (pQC30) were converted to spheroplasts and proteins detected by immunoblot. C) Immunoblots of anti-HA co-immunoprecipitation experiments as described in Materials and Methods. Totals (T) represent 5% of the solubilized input material, mock immunoprecipitations (−Ab) and anti-HA immunoprecipitations (+Ab) are labelled above each lane. Sec61p (ER) and Och1p (Golgi) serve as negative controls to demonstrate specificity of the immunoprecipitations. D) Graphical representation of the data in panel (C) showing ALP dimer formation as the ratio of HA-tagged and untagged pro-forms of ALP in lanes 9 and 12. E) The percentage of total Erv26p that co-immunoprecipitated with ALP-HA in lanes 3 and 6 normalized by the ALP-HA signals in these same lanes. The positions of pro-ALP (p), mature ALP (m), pro-ALP-HA (HA) and a soluble breakdown product (*) are indicated.
Figure 9A, the Δ79-82ALP mutant was stabilized in erv26Δ and displayed a t1/2 of ~ 35 min in wild-type and >60 min in the erv26Δ strain (Figure 9B). The lack of vacuolar cleavage even after 1 h of chase (and at steady state, Figure 8A) suggests that the degradation is prevacuolar. A decrease in the electrophoretic mobility of the Δ79-82ALP protein was observed at 30 and 60 min. We speculate that this dimerization mutant received additional post-translational modification with prolonged residence in the ER. The Δ79-82ALP protein did not efficiently receive Golgi-specific α1,6-man modifications in either background (data not shown).