Table 7.
Au(I)-catalyzed hydroamination/cyclization of N-carbamoyl allenes28
| Entrya | Substrate | Conditions | Product | Yield (%) |
ee (%) |
|---|---|---|---|---|---|
| 1 |
|
A |

|
97 | 81 |
| 2 |
|
B |

|
99 | 34 |
| 3 |
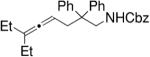
|
C |

|
83 | 91 |
| 4 |

|
A |

|
98 | 50 |
Conditions: A = 2.5 mol% [(S)-DTBM-MeOBIPHEP]Au2Cl2, 5 mol% AgClO4, 0.30 M in toluene, −40 °C, 24 h; B = same as A except −20 °C; C = same as A except 0 °C, 24 h then rt, 24 h.

