Figure 7.
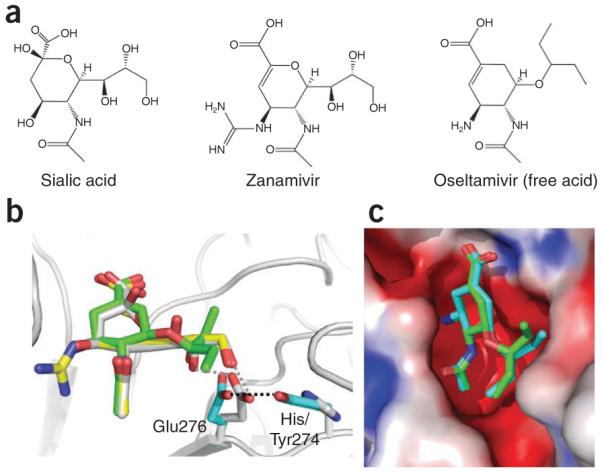
Binding of sialic acid–mimic drugs zanamivir and oseltamivir to neuraminidase (NA). (a,b) Chemical structures (a) and superposition of NA structures (b) show highly similar modes of binding for zanamivir (yellow) and oseltamivir (green) to sialic acid (gray) substrate, which reflects the influence of structures in the discovery of these drugs67,68. Recent crystal structures of oseltamivir-resistant mutant NA complexes72 show how the virus uses the NA H274Y mutation to reposition Glu276, which discriminates the l-ethylpropoxy group of oseltamivir from the glycerol moiety of the substrate. The repositioned Glu276 side chain would develop steric conflict with the l-ethylpropoxy group of oseltamivir, whereas it could still maintain favorable interactions with the glycerol part, common to both sialic acid and zanamivir. (c) Comparison of the modes of binding of oseltamivir to wild-type and H267Y mutant NA (shown in green and cyan, respectively). The H264Y mutation affects the positioning of the l-ethylpropoxy group of oseltamivir. The rearrangement of l-ethylpropoxy group of oseltamivir is associated with loss of inhibitor-protein interactions, resulting in a significant drug resistance.
