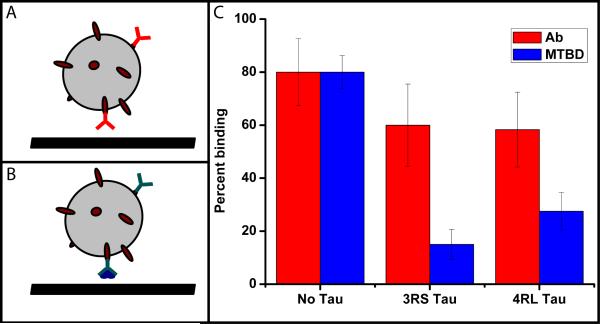
Fig. 5. Static binding experiments probe the role of tau at and away from the MT.
We tested how tau affects binding of MTBD to MTs with bead-based assay (a,b). We used polystyrene beads (schematically represented by grey spheres) conjugated to protein A (dark red ovals). Protein A was in turn bound with anti-tubulin antibody (red Y) or anti-GST antibody (cyan Y) and GST-tagged MTBD of dynein (dark blue). These two bead configurations were bound to MTs with no tau, 3RS and 4RL tau (0.21 bound tau/tubulin dimer ratio). The results of binding experiments are shown in (c). The presence of either isoform of tau on MTs only slightly reduces bead binding to MTs (red bars). However, tau very strongly inhibits the binding of MTBD of dynein (blue bars) even though the geometries used (a,b) are nearly identical. Note that the 3RS inhibits MTBD binding more, consistent with our observation that this isoform has a stronger effect on dynein on-rate and dynein-driven bead travel distances (Fig. 2, 4). Note also that the full dynein motor has two binding domains so the net effect of tau on full motor binding is likely to be less pronounced than for a single dynein’s MT-binding domain. The error bars shown are estimated as , where P is the fraction of binding events and Ntotal is the number of beads tested.