Figure 1.
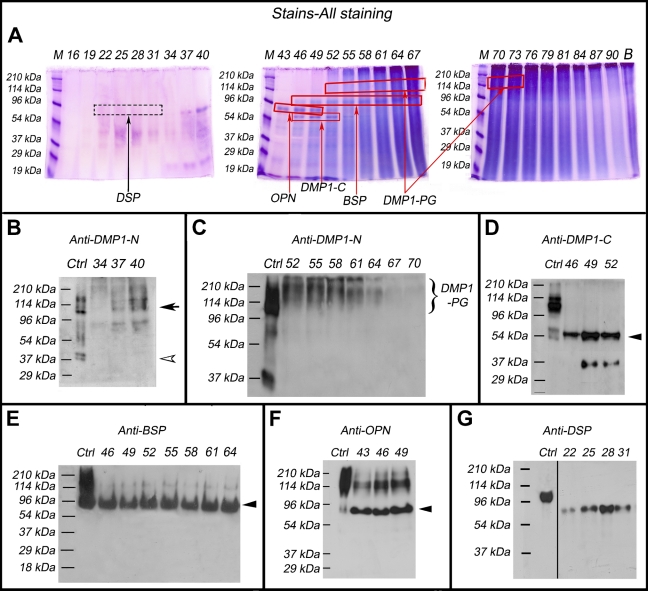
Identification of the small integrin-binding ligand, N-linked glycoprotein (SIBLING) family members in the femoral head cartilage by Stains-all staining and Western immunoblotting. Numbers at the top of each image represent fraction numbers of the Q-Sepharose chromatography. (A) Stains-all staining for every third fraction in the chromatographic region from fractions 16–90 is presented here to illustrate the existence of the four SIBLING family members. The blue protein bands in fractions 43–52 migrating between the 53- and 78-kDa molecular mass markers represent osteopontin (OPN; boxed). The dentin matrix protein 1 (DMP1)-C fragment (∼57 kDa, boxed) co-eluted with OPN in fractions 46–52. Bone sialoprotein (BSP; ∼90 kDa, boxed) mainly eluted in fractions 46–64. The identities of these SIBLING family members were confirmed by Western immunoblotting (B–G). (B) Control (Ctrl): 1 μg of DMP1 (including the full-length DMP1 and its processed fragments) extracted from long bone of rat. Western immunoblotting using the anti-DMP1-N-9B6.3 antibody demonstrated the presence of DMP1-N (37 kDa, white arrowhead) and full-length DMP1 (∼110 kDa, black arrow) in fractions 34–40. (C) Ctrl: 1 μg of DMP1 isolated from long bone of rat. Note the presence of DMP1-proteoglycan (DMP1-PG) recognized by the anti-DMP1-N-9B6.3 antibody in fractions 52–70. (D) Ctrl: 1 μg of DMP1 isolated from long bone of rat. DMP1-C (arrowhead) was detected by the anti-DMP1-C-857 antibody in fractions 46–52. (E) Ctrl: 1 μg of BSP isolated from long bone of rat. BSP (arrowhead) was recognized by the anti-BSP-10D9.2 antibody in fractions 46–64. (F) Ctrl: 1 μg of OPN isolated from long bone of rat. OPN (arrowhead) was detected by the anti-OPN antibody in fractions 43–49. (G) Ctrl: 0.3 μg of dentin sialoprotein (DSP) isolated from incisor dentin of rat. DSP was detected by the anti-DSP-2G7.3 antibody in fractions 22–31. Note that DSP extracted from the cartilage migrated faster than that isolated from rat dentin (i.e., the positive Ctrl). The lane for the positive Ctrl sample in the original Western immunoblotting gel was two lanes away from the lane of fraction 22; we horizontally moved the Ctrl lane to the position next to fraction 22 to better illustrate the results.