Fig. 8.
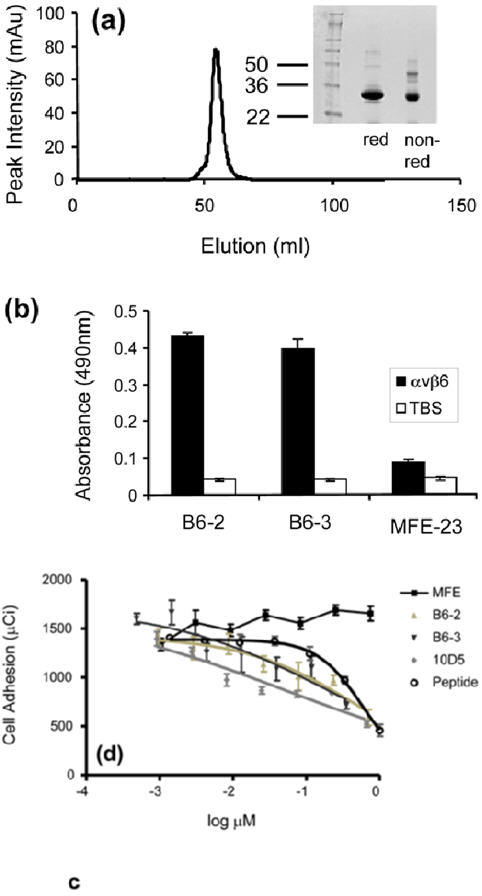
Properties of B6-3. (a) Size-exclusion chromatography profile showing that B6-3 formed a dimer. Twelve percent Tri-glycine SDS-PAGE results under reducing and non-reducing conditions are shown of the dimeric fraction. (b) Chart showing that B6-3 bound to immobilized αvβ6 in ELISA. B6-2, B6-3 or MFE-23 was applied at 20 μg/ml to immobilized αvβ6 and control Tris-buffered (TBS) wells. Binding was detected with mouse anti-Tetra-His IgG followed by sheep anti-mouse HRP-linked secondary antibody. The data represent the mean of triplicate measurements, and error bars represent the standard deviation at each data point. (c) Graph showing that B6-3 inhibited the adhesion of αvβ6-expressing cells to LAP. Radiolabeled [51Cr] 3T3β6.19 cells in various concentrations of MFE-23, B6-2, B6-3, 10D5 or the VP1 peptide A20FMDV224 were added to 96-well plates coated with 50 μl (0.25 μg/ml) LAP. Data show the mean and standard deviations of quadruplet wells. IC50 values obtained from the experiment are as follows: A20FMDV2, 589.6±101.0 nM; B6-2, 483.5±40.5 nM; B6-3, 196.2±27.6 nM; 10D5, 51.4±32.0 nM.
