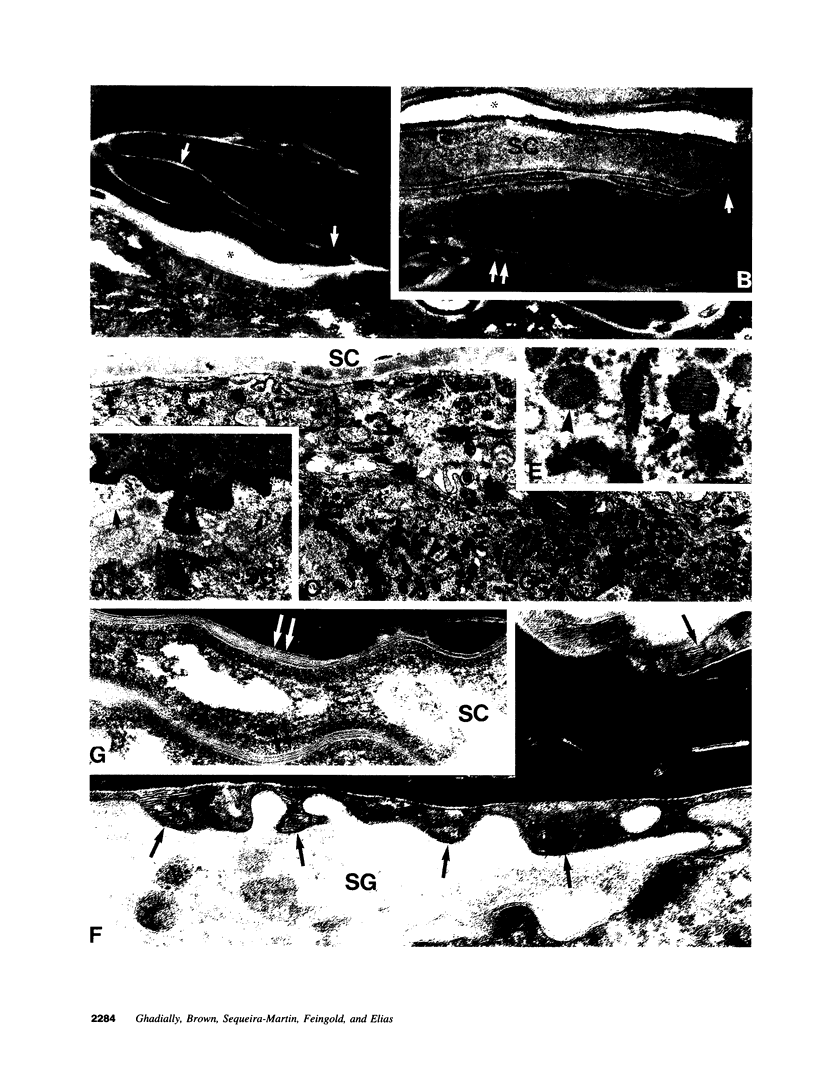
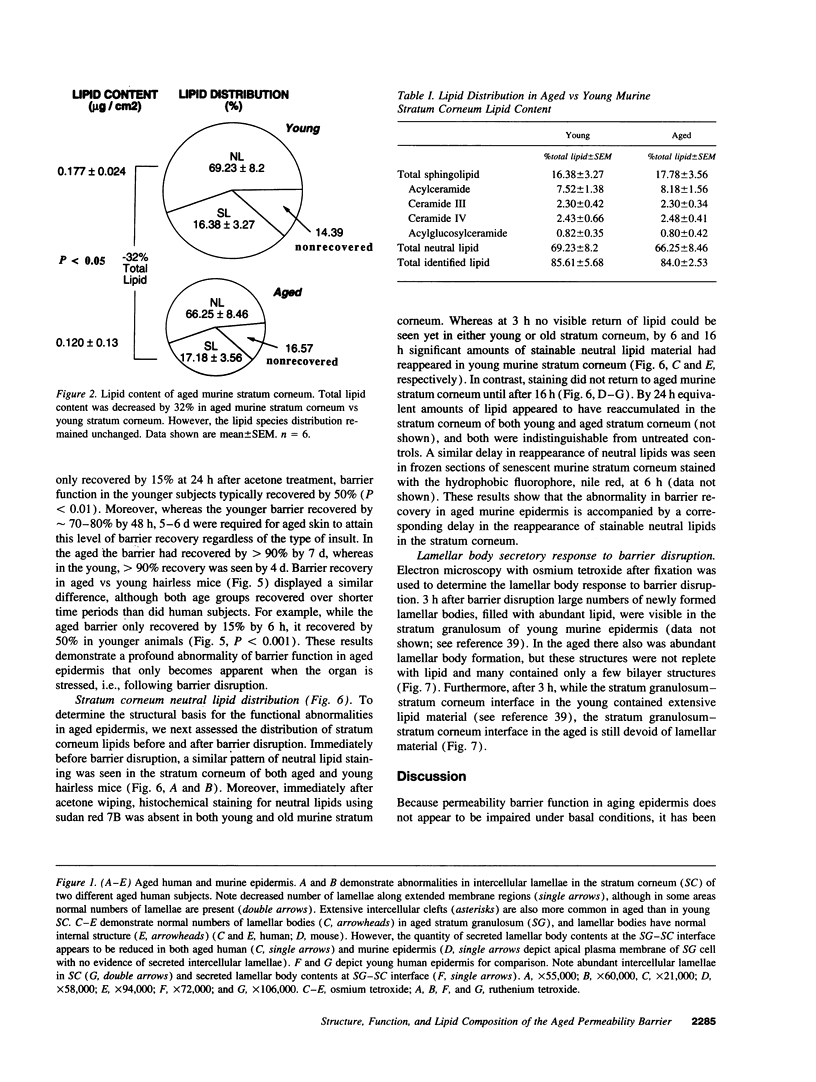
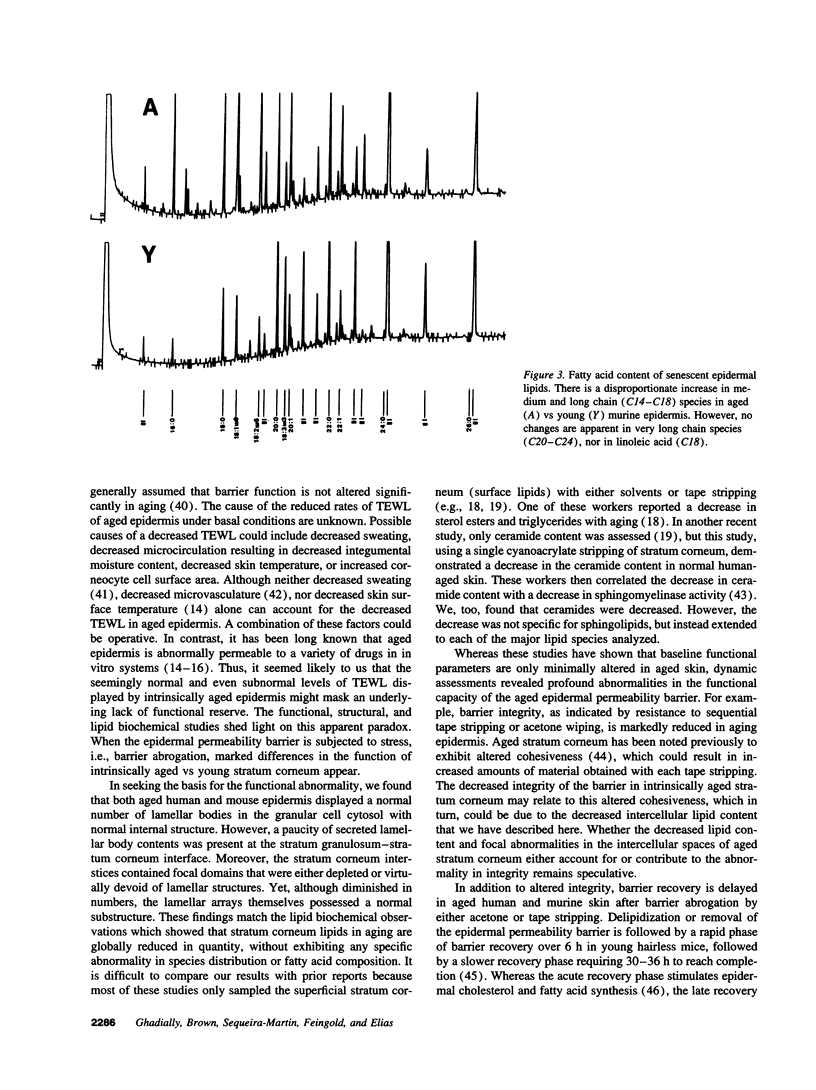
Abstract
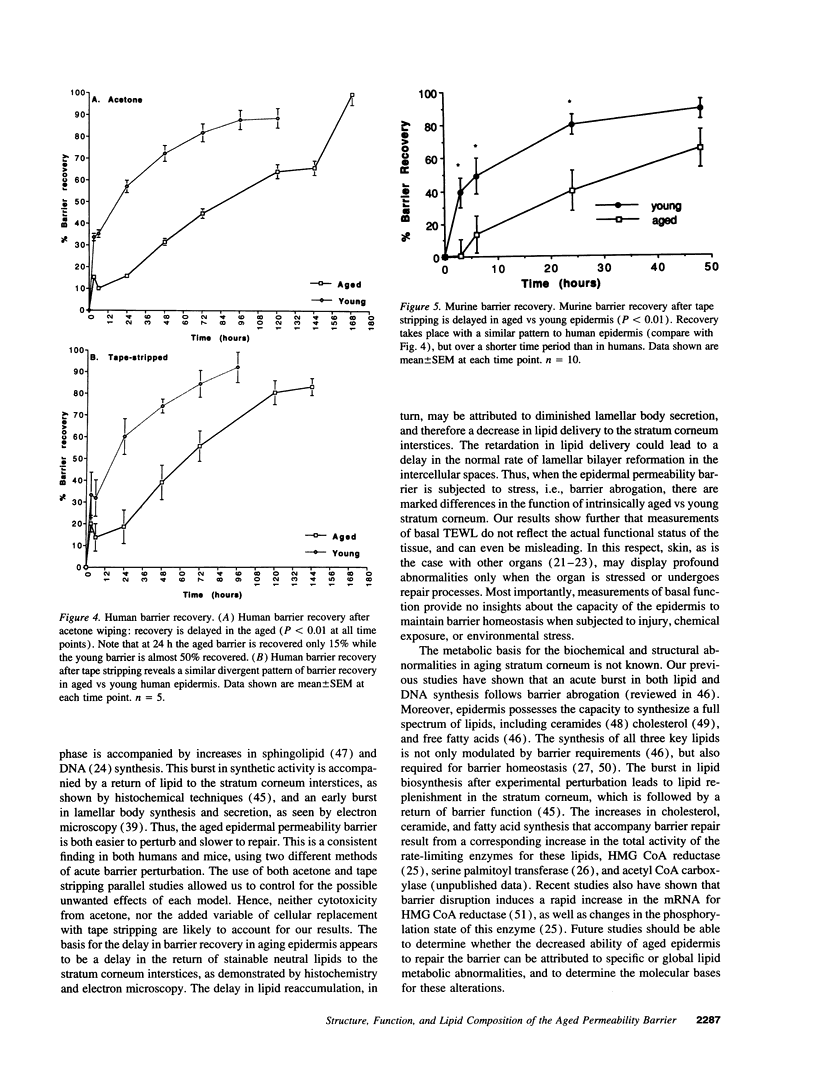
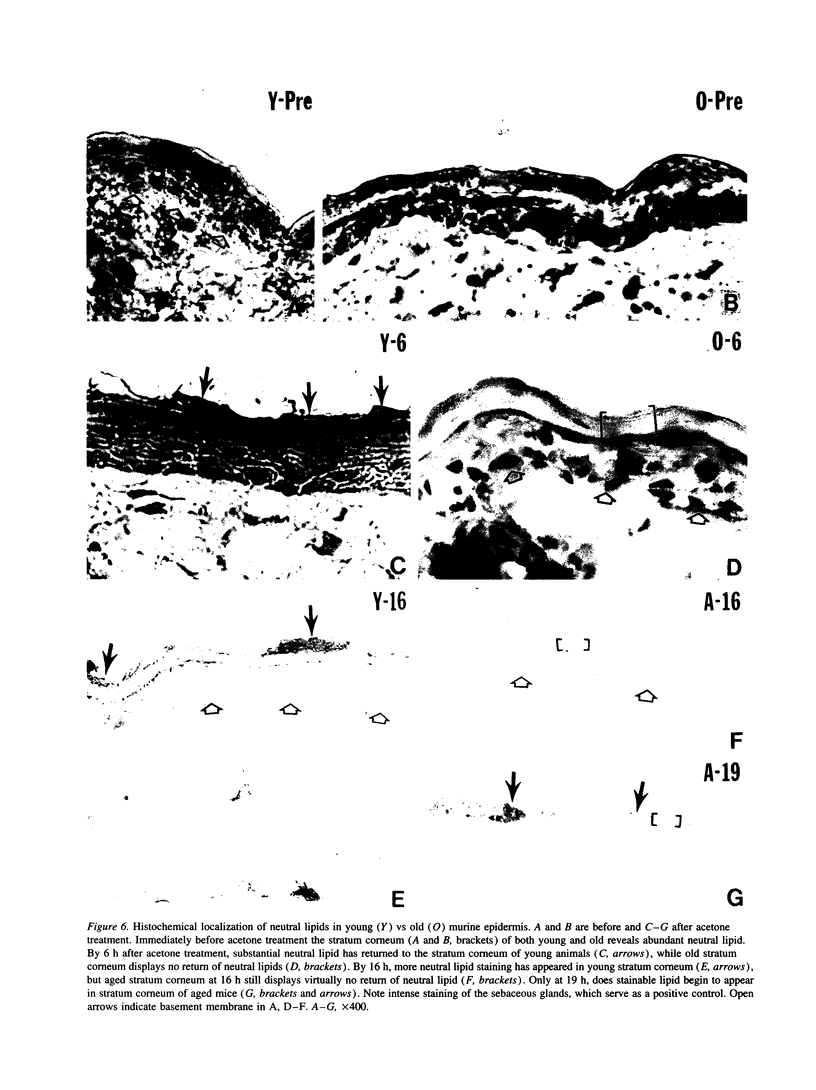
Aged epidermis displays altered drug permeability, increased susceptibility to irritant contact dermatitis, and often severe xerosis, suggesting compromise of the aged epidermal barrier. To delineate the functional, structural, and lipid biochemical basis of epidermal aging, we compared barrier function in young (20-30 yr) vs aged (> 80 yr) human subjects, and in a murine model. Baseline transepidermal water loss in both aged humans and senescent mice was subnormal. However, the aged barrier was perturbed more readily with either acetone or tape stripping (18 +/- 2 strippings vs 31 +/- 5 strippings in aged vs young human subjects, respectively). Moreover, after either acetone treatment or tape stripping, the barrier recovered more slowly in aged than in young human subjects (50 and 80% recovery at 24 and 72 h, respectively, in young subjects vs 15% recovery at 24 h in aged subjects), followed by a further delay over the next 6 d. Similar differences in barrier recovery were seen in senescent vs young mice. Although the total lipid content was decreased in the stratum corneum of aged mice (approximately 30%), the distribution of ceramides (including ceramide 1), cholesterol, and free fatty acids was unchanged. Moreover, a normal complement of esterified, very long-chain fatty acids was present. Finally, stratum corneum lamellar bilayers displayed normal substructure and dimensions, but were focally decreased in number, with decreased secretion of lamellar body contents. Thus, assessment of barrier function in aged epidermis under basal conditions is misleading, since both barrier integrity and barrier repair are markedly abnormal. These functional changes can be attributed to a global deficiency in all key stratum corneum lipids, resulting in decreased lamellar bilayers in the stratum corneum interstices. This constellation of findings may explain the increased susceptibility of intrinsically aged skin to exogenous and environmental insults.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Black M. M. A modified radiographic method for measuring skin thickness. Br J Dermatol. 1969 Sep;81(9):661–666. doi: 10.1111/j.1365-2133.1969.tb16204.x. [DOI] [PubMed] [Google Scholar]
- Blichmann C. W., Serup J. Reproducibility and variability of transepidermal water loss measurement. Studies on the Servo Med Evaporimeter. Acta Derm Venereol. 1987;67(3):206–210. [PubMed] [Google Scholar]
- Cua A. B., Wilhelm K. P., Maibach H. I. Frictional properties of human skin: relation to age, sex and anatomical region, stratum corneum hydration and transepidermal water loss. Br J Dermatol. 1990 Oct;123(4):473–479. doi: 10.1111/j.1365-2133.1990.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Elias H., Hennig A., Schwartz D. E. Stereology: applications to biomedicalresearch. Physiol Rev. 1971 Jan;51(1):158–200. doi: 10.1152/physrev.1971.51.1.158. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Brown B. E., Fritsch P., Goerke J., Gray G. M., White R. J. Localization and composition of lipids in neonatal mouse stratum granulosum and stratum corneum. J Invest Dermatol. 1979 Nov;73(5):339–348. doi: 10.1111/1523-1747.ep12550377. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Feingold K. R. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992 Jun;11(2):176–182. [PubMed] [Google Scholar]
- Elsner P., Wilhelm D., Maibach H. I. Frictional properties of human forearm and vulvar skin: influence of age and correlation with transepidermal water loss and capacitance. Dermatologica. 1990;181(2):88–91. doi: 10.1159/000247892. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Brown B. E., Lear S. R., Moser A. H., Elias P. M. Localization of de novo sterologenesis in mammalian skin. J Invest Dermatol. 1983 Oct;81(4):365–369. doi: 10.1111/1523-1747.ep12519974. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Man M. Q., Menon G. K., Cho S. S., Brown B. E., Elias P. M. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990 Nov;86(5):1738–1745. doi: 10.1172/JCI114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S. D., Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985 Aug;33(8):833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- Ghadially R., Halkier-Sorensen L., Elias P. M. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992 Mar;26(3 Pt 2):387–396. doi: 10.1016/0190-9622(92)70060-s. [DOI] [PubMed] [Google Scholar]
- Grove G. L., Duncan S., Kligman A. M. Effect of ageing on the blistering of human skin with ammonium hydroxide. Br J Dermatol. 1982 Oct;107(4):393–400. doi: 10.1111/j.1365-2133.1982.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Grubauer G., Elias P. M., Feingold K. R. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989 Mar;30(3):323–333. [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Harris R. M., Elias P. M. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989 Jan;30(1):89–96. [PubMed] [Google Scholar]
- Holleran W. M., Feingold K. R., Man M. Q., Gao W. N., Lee J. M., Elias P. M. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res. 1991 Jul;32(7):1151–1158. [PubMed] [Google Scholar]
- Holleran W. M., Man M. Q., Gao W. N., Menon G. K., Elias P. M., Feingold K. R. Sphingolipids are required for mammalian epidermal barrier function. Inhibition of sphingolipid synthesis delays barrier recovery after acute perturbation. J Clin Invest. 1991 Oct;88(4):1338–1345. doi: 10.1172/JCI115439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S. Y., Mitra A. K., White S. H., Menon G. K., Ghadially R., Elias P. M. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991 Feb;96(2):215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Imokawa G., Abe A., Jin K., Higaki Y., Kawashima M., Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991 Apr;96(4):523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- Jackson S. M., Wood L. C., Lauer S., Taylor J. M., Cooper A. D., Elias P. M., Feingold K. R. Effect of cutaneous permeability barrier disruption on HMG-CoA reductase, LDL receptor, and apolipoprotein E mRNA levels in the epidermis of hairless mice. J Lipid Res. 1992 Sep;33(9):1307–1314. [PubMed] [Google Scholar]
- Lampe M. A., Williams M. L., Elias P. M. Human epidermal lipids: characterization and modulations during differentiation. J Lipid Res. 1983 Feb;24(2):131–140. [PubMed] [Google Scholar]
- Leveque J. L., Corcuff P., de Rigal J., Agache P. In vivo studies of the evolution of physical properties of the human skin with age. Int J Dermatol. 1984 Jun;23(5):322–329. doi: 10.1111/j.1365-4362.1984.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Long C. C., Marks R. Stratum corneum changes in patients with senile pruritus. J Am Acad Dermatol. 1992 Oct;27(4):560–564. doi: 10.1016/0190-9622(92)70222-2. [DOI] [PubMed] [Google Scholar]
- MALKINSON F. D. Studies on the percutaneous absorption of C14 labeled steroids by use of the gas-flow cell. J Invest Dermatol. 1958 Jul;31(1):19–28. doi: 10.1038/jid.1958.71. [DOI] [PubMed] [Google Scholar]
- Marks R. Measurement of biological ageing in human epidermis. Br J Dermatol. 1981 Jun;104(6):627–633. doi: 10.1111/j.1365-2133.1981.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Elias P. M. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992 Mar;98(3):279–289. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Moser A. H., Brown B. E., Elias P. M. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985 Apr;26(4):418–427. [PubMed] [Google Scholar]
- Nilsen K. H. Reactive hyperemia in scleroderma. J Invest Dermatol. 1979 Sep;73(3):259–259. doi: 10.1111/1523-1747.ep12514495. [DOI] [PubMed] [Google Scholar]
- Pinnagoda J., Tupker R. A., Agner T., Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990 Mar;22(3):164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Ponec M., Weerheim A., Kempenaar J., Mommaas A. M., Nugteren D. H. Lipid composition of cultured human keratinocytes in relation to their differentiation. J Lipid Res. 1988 Jul;29(7):949–961. [PubMed] [Google Scholar]
- Potts R. O., Buras E. M., Jr, Chrisman D. A., Jr Changes with age in the moisture content of human skin. J Invest Dermatol. 1984 Jan;82(1):97–100. doi: 10.1111/1523-1747.ep12259203. [DOI] [PubMed] [Google Scholar]
- Proksch E., Elias P. M., Feingold K. R. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in murine epidermis. Modulation of enzyme content and activation state by barrier requirements. J Clin Invest. 1990 Mar;85(3):874–882. doi: 10.1172/JCI114514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch E., Feingold K. R., Man M. Q., Elias P. M. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991 May;87(5):1668–1673. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskos K. V., Maibach H. I. Percutaneous absorption and age. Implications for therapy. Drugs Aging. 1992 Sep-Oct;2(5):432–449. doi: 10.2165/00002512-199202050-00007. [DOI] [PubMed] [Google Scholar]
- Saint Léger D., François A. M., Lévêque J. L., Stoudemayer T. J., Grove G. L., Kligman A. M. Age-associated changes in stratum corneum lipids and their relation to dryness. Dermatologica. 1988;177(3):159–164. doi: 10.1159/000248535. [DOI] [PubMed] [Google Scholar]
- Shuster S., Black M. M., McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975 Dec;93(6):639–643. doi: 10.1111/j.1365-2133.1975.tb05113.x. [DOI] [PubMed] [Google Scholar]
- Thune P. Evaluation of the hydration and the water-holding capacity in atopic skin and so-called dry skin. Acta Derm Venereol Suppl (Stockh) 1989;144:133–135. doi: 10.2340/00015555144133135. [DOI] [PubMed] [Google Scholar]
- Wilhelm K. P., Cua A. B., Maibach H. I. Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol. 1991 Dec;127(12):1806–1809. doi: 10.1001/archderm.127.12.1806. [DOI] [PubMed] [Google Scholar]