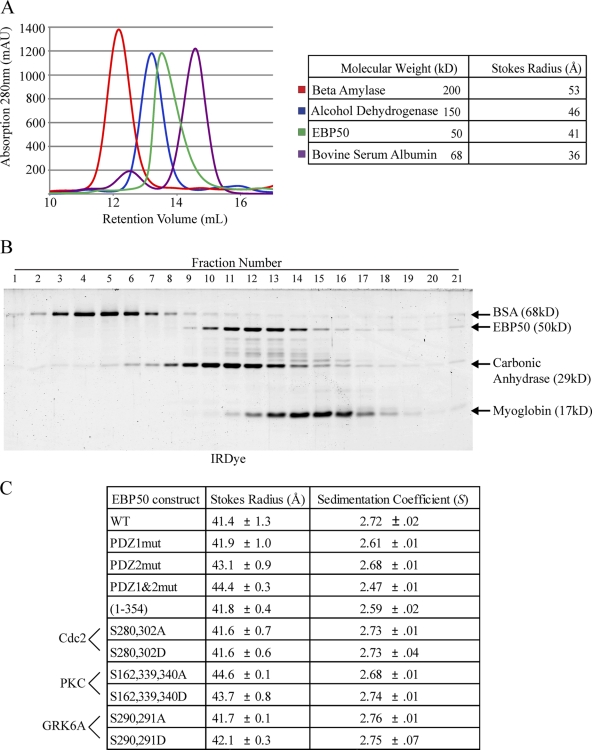
Figure 8.
EBP50 is an elongated monomer. (A) Untagged, full-length EBP50 was run over a Superdex 200 10/300 GL column and compared with size standards β-amylase, alcohol dehydrogenase, and bovine serum albumin (BSA); the Stokes radius of EBP50 was calculated to be 41 Å. (B) EBP50 was run on sucrose gradients with size standards BSA (4.30 S), carbonic anhydrase (3.20 S), and myoglobin (2.04 S); the sedimentation coefficient was calculated to be 2.72 S. (C) Table summarizing gel filtration and sucrose gradient results for all PDZ and phosphorylation mutants of EBP50. The results are from at least two independent determinations each.