Figure 4.
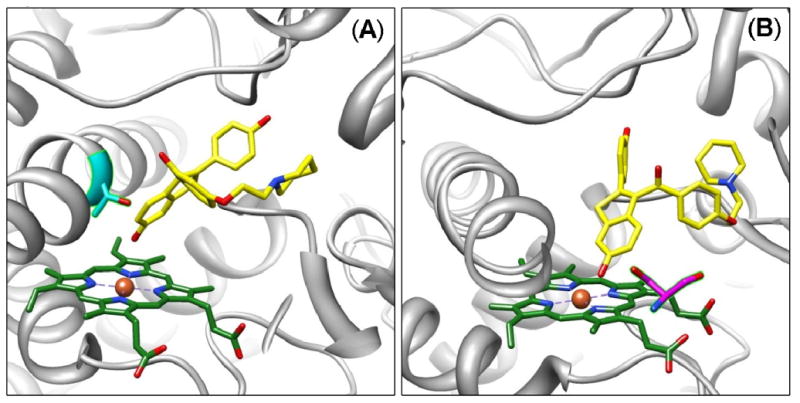
Molecular models of raloxifene in the active site of 1W0E (without heme partial charges) predicted by AutoDock 3.0. (A) A conformation predicting dehydrogenation of raloxifene, where the active site Thr309 residue (cyan) is in position to donate a hydrogen bond to the oxygen of the hydroxyl group on the benzothiophene moiety of raloxifene. (B) A conformation predicting dehydrogenation of raloxifene, where the active site Ser119 residue (magenta) is in position to donate a hydrogen bond to the oxygen of the hydroxyl group on the benzothiophene moiety of raloxifene. CYP3A4 is shown in a ribbon format, iron as a sphere, heme without partial charges (green) and docking raloxifene (yellow) in color-coded sticks: nitrogen = blue, oxygen = red. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco.
