Figure 5.
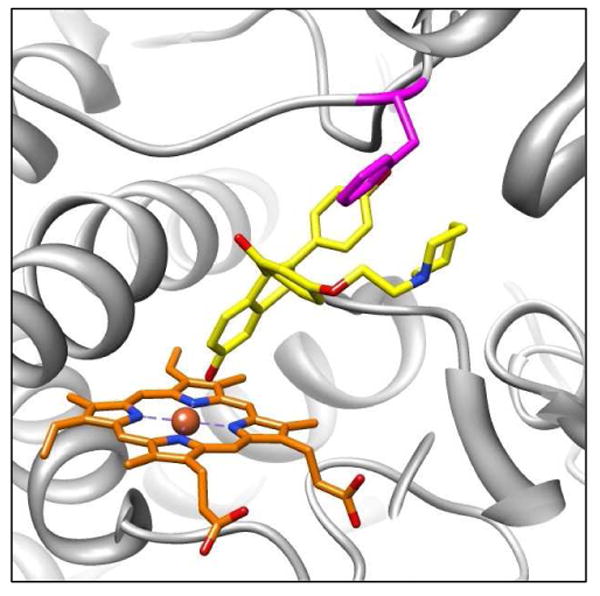
Molecular models of raloxifene in the active site of 1W0E_Modified (with partial charges assigned to the heme) predicted by AutoDock 3.0. A representative conformation from the largest/lowest energy cluster predicting the dehydrogenation of raloxifene. The active site Phe215 residue (magenta) is in position to orient raloxifene by steric interactions and π bond T-stacking between raloxifene's phenol moiety and Phe215's benzene ring. CYP3A4 is shown in a ribbon format, iron as a sphere, heme with partial charges (orange) and docking raloxifene (yellow) in color-coded sticks: nitrogen = blue, oxygen = red. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco.
