Abstract
A 23-year-old woman without history of antecedent vocal, respiratory, or sleep disorders received vagus nerve stimulation (VNS) therapy for refractory partial epilepsy and developed sleep-related stridor during the course of parameter titration. Reduction of VNS current during polysomnography completely eliminated stridor. We conclude that VNS may cause sleep-related stridor in rare cases, expanding the spectrum of known sleep-disordered breathing disorders associated with VNS therapy. Parameter adjustment during polysomnography may resolve nocturnal stridor caused by VNS.
Keywords: epilepsy, sleep, VNS, stridor, breathing, polysomnogram
Vagus nerve stimulation (VNS) is approved for adjunctive treatment of refractory partial onset-seizures in adolescents and adults. VNS therapy improves daytime alertness in some patients (Malow et al., 2001; Galli et al., 2003), but conversely also produces excessive daytime sleepiness and causes obstructive or central sleep apnoea in others (Holmes et al., 2003; Malow et al., 2000; Marzec et al., 2003; Nagarajan et al., 2003; Papacostas et al., 2007; Rychlicki et al., 2006) Sleep-disordered breathing associated with VNS may in turn be improved by altering VNS stimulation parameters (Holmes et al., 2003; Malow et al., 2000; Marzec et al., 2003). To our knowledge, no previous case of VNS-induced nocturnal stridor has been reported.
Case history
A 23-year-old woman without previous history of respiratory or sleep complaints underwent VNS implantation for medically refractory non-lesional extratemporal partial epilepsy. Habitual clinical seizures began at age five with complex partial and secondarily generalised tonic-clonic seizures occurring in clusters four times monthly. Birth and developmental history were remarkable for precocious puberty resolved by leuprolide acetate (Lupron) therapy, and a perinatal subarachnoid haemorrhage with a single brief symptomatic convulsive seizure on the first day of life. Evaluation prior to VNS implantation included a volumetric brain MRI which demonstrated right mesial occipital encephalomalacia with normal hippocampi, and ictal video-EEG monitoring which recorded numerous poorly localized complex partial and secondarily generalised tonic-clonic seizures with independent left or right hemispheric onsets. Medical therapy included lamotrigine 200 mg BID, paroxetine 30 mg daily for comorbid depression, and a prenatal multivitamin, and previous failed medical therapies included phenobarbital, carbamazepine, gabapentin, levetiracetam, and topiramate.
During the first six months of VNS therapy at device output currents adjusted up to 0.5 milliamps, she reported no symptoms of sleep-disordered breathing. Due to continued seizures, her device output current was further titrated to 0.75 milliamps, but shortly following application of this new setting, her family reported hearing loud snoring, and the patient noted new symptoms of mild daytime sleepiness. Physical examination showed significant oropharyngeal crowding (Friedman Palate Position Score of IV; Friedman et al., 1999), neck circumference of 14 inches (35.5 cm), and body mass index of 35.4 kg/m2. The Epworth Sleepiness Scale (ESS) score was 7. Polysomnography demonstrated mild primary snoring with an apnoea-hypopnoea index of 0. Following subsequent VNS output current titration to 1.0 milliamp, seizure frequency was reduced but the patient’s family then reported hearing a new loud, peculiar respiratory noise exclusively during sleep that recurred every few minutes throughout the night. She denied intermittent dysarthria or dysphagia. Repeat physical examination demonstrated no vocal hoarseness during periods of VNS duty cycle activation. There was no audible wheezing or adventitious pulmonary or tracheal sounds during daytime auscultation, and no extrapyramidal or pyramidal abnormalities on neurological examination. VNS pulse width was reduced from 500 to 250 msec and signal frequency decreased from 30 to 20 hertz, without improvement of the patient’s snoring or nocturnal respiratory noises. A repeat ESS score was 9.
Another polysomnogram was subsequently performed, and table 1 displays relevant tabulated data from the study. The repeat study documented intermittent, cyclic stridor during inspiration and expiration that recurred regularly through the first part of the night, coinciding with VNS duty cycle (see video sequence, figure 1). The apnoea-hypopnoea index was again 0, and while there were otherwise asymptomatic airflow limitations corresponding with the audible stridor, there were no clear respiratory effort-related arousals or oxyhaemoglobin desaturations. VNS reprogramming was performed during the study that evening in the sleep laboratory by the treating epileptologist (EKS). Snoring and stridor halted completely during VNS deactivation, recurred immediately following resumed VNS, and sequential reductions of VNS output current to 0.5 milliamps completely obviated stridor and snoring (figure 2). VNS duty cycle was shortened to 21 seconds “on” and 0.8 minutes “off”, to more adequately treat seizures. Flexible fibreoptic laryngoscopic examination of the nasopharynx, larynx, sub-glottis, and upper trachea was normal. Two years later, snoring, stridor, and daytime sleepiness have not recurred, and there has been a 50% reduction in seizure frequency.
Table 1.
Polysomnogram statistical summary data.
| Recording time | 9 hours 53 minutes |
| Total sleep time | 9 hours 24 minutes |
| Sleep efficiency | 95.3% |
| Initial sleep latency | 9.0 minutes |
| Initial REM latency | 349.0 minutes |
| Sleep stage percentages | |
| - N1 | 10.4% |
| - N2 | 60.8% |
| - N3 | 15.3% |
| - REM | 13.5% |
| Oxyhaemoglobin saturation | |
| - Mean | 98% |
| - Nadir | 96% |
| Apnoea-hypopnoea index | 0/hr |
| Arousal index | 5.6/hr |
| Periodic limb movement | 3.4/hr |
| Index | 1.7/hr |
| - Movement arousal index | |
Figure 1.
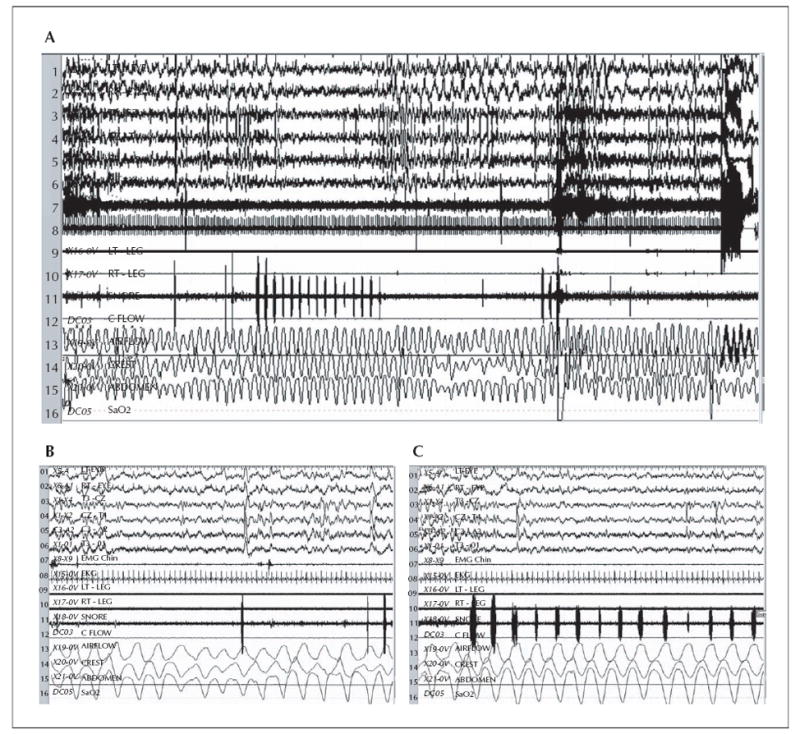
Polysomnogram shows stridor in the sonogram (channel 11) during 5-minute (A) and two consecutive 60-second epochs (B, C). During N2 sleep, recurrent periods of stridor were consistently demonstrated as deflections in the “Snore” Channel 11. Mild transient oronasal airflow limitation and altered respiratory effort occurred in relationship to the audible stridor without associated arousal or oxyhaemoglobin desaturation (A, B). A spontaneous arousal is seen in the latter one-third section of A, but no clearly corresponding respiratory effort-related arousals (RERAs) were seen during or following periods of stridor, and no oxyhaemoglobin desaturations occurred. Arousal index was within normal limits at 5.6 per hour, suggesting that upper airway resistance syndrome was unlikely. Sensitivity 15 uV/mm, TC 0.3 sec, HFF 35 hertz.
Figure 2.
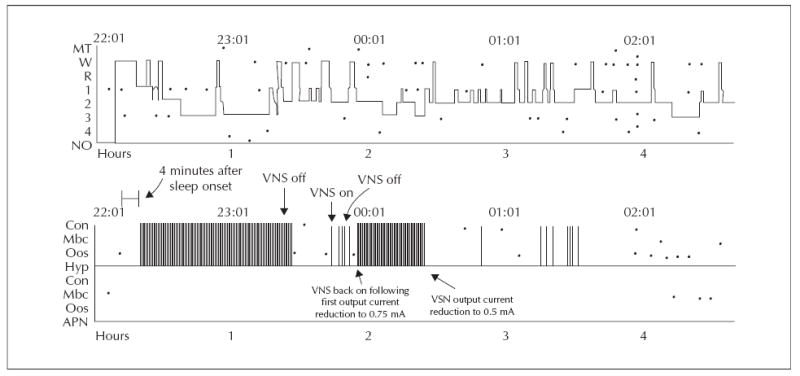
Hypnogram depicting course of sleep-related stridor during VNS parameter adjustment. Sleep-related stridor/snoring was initially frequent, coinciding with VNS duty cycle, was completely resolved by VNS deactivation, recurred with reactivation and was then resolved following VNS parameter adjustment (reduced stimulus intensity).
Discussion
VNS produced sleep-related stridor in our patient, expanding the known spectrum of sleep-disordered breathing disorders caused by VNS therapy. Notably, sleep-related stridor emerged during VNS current titration in the absence of commonly encountered daytime adverse effects such as pharyngeal discomfort, vocal hoarseness, dysphagia, or dyspnoea (Handforth et al., 1998). Daytime vocal hoarseness is a particularly common consequence of upward titration of output current, and may occur during each stimulation train with prompt reversibility by reduction of the output current setting (Ben-Menachem, 2001). Similar to these daytime symptoms and signs of VNS over-stimulation, sleep-related stridor was fully and quickly reversible in our case by modulation of VNS output current, demonstrating that dynamic in-lab adjustment of VNS stimulation parameters during polysomnography is feasible and effective in exceptional appropriately selected patients.
Stridor is a harsh, high-pitched sound due to turbulent flow in the upper airway that may portend acute upper airway compromise. Stridor during wakefulness is a manifold disorder caused by a variety of local laryngeal or tracheal pathologies. However, sleep-related stridor is relatively rare and considered most characteristic of multiple systems atrophy (MSA), a neurodegenerative extra-pyramidal disorder involving parkinsonism poorly responsive to levodopa, cerebellar ataxia, upper motor neuron signs, and autonomic failure (Bhidayasiri and Ling, 2008). Sleep-related stridor may herald the diagnosis of MSA, and has in that context been linked to an unpredictable risk of sudden death (Silber and Levine, 2000). While we doubted the possibility of MSA in our patient given her youth and absence of clinical signs of parkinsonism or dysautonomia, we nevertheless felt that prompt VNS adjustment to eliminate audible stridor was indicated, given the recognized dangers of untreated nocturnal stridor, as well as the family’s concern about the patient’s sleep-related breathing.
We also reasoned, although could not prove, that her recently evolved mild hypersomnia may have represented an upper airway resistance syndrome (UARS) variant of mild obstructive sleep apnoea (OSA), or isolated supine REM OSA. The possibility of clinically disruptive sleep-disordered breathing was further suggested by evolution of mild hypersomnia symptoms during VNS current titration that later resolved with current reduction, although we were unable to further substantiate that possibility given that no clear apnoeas, hypopnoeas, or respiratory effort-related arousals (RERAs) were seen during polysomnography. We did note several periods of intermittent airflow limitation unassociated with arousal or oxyhaemoglobin desaturation that coincided with periods of audible stridor, similar to previously reported asymptomatic airflow limitations occurring during periods of VNS on-stimulation cycles (Gschliesser et al., 2009; Malow et al., 2000; Marzec et al., 2003; Nagarajan et al., 2003). However, since supine REM sleep was not recorded during our patient’s study, it is conceivable that isolated OSA during supine REM was missed, or that UARS was missed given that our laboratory does not utilize oesophageal manometry, a technique that may be superior for distinguishing subtle RERAS from spontaneous arousals. Our patient’s normal arousal index of only 5.6/hour would appear to be evidence against UARS. Her mild hypersomnia could also have been the consequence of chronically disturbed sleep architecture given the findings of a reduced REM percentage and delayed REM latency during polysomnography. While these findings are perhaps best attributed to concurrent paroxetine therapy, recent seizures or lamotrigine administration may also have mediated disturbances in sleep architecture in our patient, as previously suggested (Bazil, 2003).
Our decision to lower VNS current, exposing the patient to a risk of deterioration in seizure control, could certainly be reasonably debated, since polysomnography showed no obvious or prominent respiratory disturbance aside from audible stridor. Fortunately, augmentation of other VNS stimulation parameters instead lead to further improvement in seizure control. The patient and her family remain pleased with her improved daytime alertness, absence of recurrent nocturnal stridor, and improved seizure control at reduced VNS output current and rapid cycle stimulation. The impact of VNS on sleep respiration has not been widely studied. Reductions in airflow, tidal volume, and respiratory effort, as well as increased or decreased respiratory rate, have been documented in adults and children following VNS implantation, chiefly during the on-time stimulation cycle, and apnoeic events have been successfully resolved with parameter alteration or nasal CPAP therapy in most cases (Gschliesser et al., 2009; Holmes et al., 2003; Malow et al., 2000; Marzec et al., 2003; Nagarajan et al., 2003). Two recently reported cases have suggested that CPAP may be ineffective for relief of OSA during periods of active VNS stimulation; in such cases, alternative tactics reported to successfully relieve refractory VNS-mediated OSA include temporary or permanent VNS deactivation, or injection of botulinum toxin if VNS-induced laryngospasm can be confirmed (Ebben et al., 2008; Kumar et al., 2009). Adjustment of VNS stimulation parameters rapidly obviated our patient’s stridor. Reduction of output current was necessary to improve stridor in our patient, distinct from a previous report of VNS-mediated OSA resolved by modulating stimulus frequency (without adjustments of device stimulus intensity, on time, or pulse width) (Marzec et al., 2003). Our patient’s stridor was clearly current dose-dependent and was only improved by reduction of stimulus intensity.
Mechanisms of sleep-disordered breathing during VNS are potentially multifactorial. VNS afferent modulation of dorsal medullary respiratory centres may alter respiratory function (Nagarajan et al., 2003). Clinical stridor in our patient and recorded oesophageal manometry pressure crescendos in another case (Marzec et al., 2003) are consistent with a mechanism of VNS-mediated sleep-disordered breathing caused by direct activation of vagal efferents innervating pharyngeal constrictors and/or laryngeal vocal folds. However, another possible mechanism could be VNS-induced activation of vagal afferents synapsing on the dorsal medullary vagal complex including nucleus tractus solitarius, mediating dysfunction of the central control of vagal branchiomotor efferents and resultant paradoxical contraction of laryngeal adductors during inspiration similar to adductor dysphonia seen in MSA-associated, sleep-related stridor (Merlo et al., 2002; Vetrugno et al., 2007). The recent report of directly visualized VNS-induced laryngospasm during laryngoscopy subsequently relieved by botulinum toxin injection of the left thyroarytenoid muscle supports hypotheses of VNS-induced local efferent or central modulation of vocal fold functioning (Kumar et al., 2009).
VNS caused sleep-related stridor in our patient in the absence of other significant daytime signs of over-stimulation such as vocal hoarseness, dysphagia, or dyspnoea, signalling the necessity for patients, their families, and clinicians alike to remain vigilant for symptoms of sleep-disordered breathing during VNS titration. Patients with oropharyngeal crowding, obesity, and a history of loud snoring and hypersomnia should be studied with polysomnography to exclude significant sleep-disordered breathing. VNS parameter adjustment during polysomnography may improve nocturnal stridor and sleepdisordered breathing caused by VNS.
Legend for video sequence.
Patient video recorded during polysomnogram documented intermittent, cyclic stridor during inspiration and expiration that recurred regularly through the first part of the night, coinciding with VNS duty cycle. While transient brief nasal airflow reductions with altered breathing effort could be seen as shown in figure 1A, no corresponding arousals or oxyhaemoglobin desaturations occurred. VNS reprogramming during the study that evening in the sleep lab eliminated stridor.
Acknowledgments
We are grateful for the patient’s willingness to share her sleep study recording, for the assistance of Daniel L. Herold, R.PSGT, Mayo Center for Sleep Medicine, in preparing the video segment for publication, and for secretarial assistance in manuscript preparation from Ms. Laura Disbrow, Mayo Clinic Department of Neurology.
Footnotes
Disclosure. Dr. Kevin Faber does not have any conflicts of interest to disclose. Dr. Erik St. Louis received research grant support from NIH K12 #510 17 3220 04000 11812015 during the timeframe of this study. No other conflicts of interest exist.
References
- Bazil CW. Epilepsy and sleep disturbance. Epilepsy Behav. 2003;4(Suppl. 2):S39–45. doi: 10.1016/j.yebeh.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E. Practical Considerations and Recommendations. In: Schachter SC, Schmidt D, editors. Vagus Nerve Stimulation. Vol. 69 Martin Dunitz; 2001. [Google Scholar]
- Bhidayasiri R, Ling H. Multiple system atrophy. Neurologist. 2008;14:224–37. doi: 10.1097/NRL.0b013e318167b93f. [DOI] [PubMed] [Google Scholar]
- Ebben MR, Sethi NK, Conte M, Pollak CP, Labar D. Vagus nerve stimulation, sleep apnea, and CPAP titration. Journal Clin Sleep Med. 2008;4:471–3. [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109:1901–7. doi: 10.1097/00005537-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Galli R, Bonanni E, Pizzanelli C, et al. Daytime vigilance and quality of life in epileptic patients treated with vagus nerve stimulation. Epilepsy Behav. 2003;4:185–91. doi: 10.1016/s1525-5050(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Gschliesser V, Hogl B, Frauscher B, Brandauer E, Poewe W, Luef G. Mode of vagus nerve stimulation differentially affects sleep related breathing in patients with epilepsy. Seizure. 2009;18:339–42. doi: 10.1016/j.seizure.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized, active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chang M, Kapur V. Sleep apnea and excessive daytime somnolence induced by vagal nerve stimulation. Neurology. 2003;61:1126–9. doi: 10.1212/01.wnl.0000086812.62554.06. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sharafkhaneh A, Edmonds J, Schultz R, Hopkins B. Treatment of VNS-induced laryngospasm with botulinum toxin. Neurology. 2009;73:1808–10. doi: 10.1212/WNL.0b013e3181c34ae3. [DOI] [PubMed] [Google Scholar]
- Malow BA, Edwards J, Marzec M, Sagher O, Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000;55:1450–4. doi: 10.1212/wnl.55.10.1450. [DOI] [PubMed] [Google Scholar]
- Malow BA, Edwards J, Marzec M, Sagher O, Ross D, Fromes G. Vagus nerve stimulation reduces daytime sleepiness in epilepsy patients. Neurology. 2001;57:879–84. doi: 10.1212/wnl.57.5.879. [DOI] [PubMed] [Google Scholar]
- Marzec M, Edwards J, Sagher O, Fromes G, Malow BA. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003;44:930–5. doi: 10.1046/j.1528-1157.2003.56202.x. [DOI] [PubMed] [Google Scholar]
- Merlo IM, Occhini A, Pacchetti C, Alfonsi E. Not paralysis, but dystonia causes stridor in multiple system atrophy. Neurology. 2002;58:649–52. doi: 10.1212/wnl.58.4.649. [DOI] [PubMed] [Google Scholar]
- Nagarajan L, Walsh P, Gregory P, Stick S, Maul J, Ghosh S. Respiratory pattern changes in sleep in children on vagal nerve stimulation for refractory epilepsy. Can J Neurol Sci. 2003;30:224–7. doi: 10.1017/s0317167100002638. [DOI] [PubMed] [Google Scholar]
- Papacostas SS, Myrianthopoulou P, Dietis A, Papathanasiou ES. Induction of central-type sleep apnea by vagus nerve stimulation. Electromyogr Clin Neurophysiol. 2007;47:61–3. [PubMed] [Google Scholar]
- Rychlicki F, Zamponi N, Cesaroni E, et al. Complications of vagal nerve stimulation for epilepsy in children. Neurosurg Rev. 2006;29:103–7. doi: 10.1007/s10143-005-0005-5. [DOI] [PubMed] [Google Scholar]
- Silber MH, Levine S. Stridor and death in multiple system atrophy. Mov Disord. 2000;15:699–704. doi: 10.1002/1531-8257(200007)15:4<699::aid-mds1015>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Vetrugno R, Liguori R, Cortelli P, et al. Sleep-related stridor due to dystonic vocal cord motion and neurogenic tachypnea/tachycardia in multiple system atrophy. Mov Disord. 2007;22:673–8. doi: 10.1002/mds.21384. [DOI] [PubMed] [Google Scholar]


