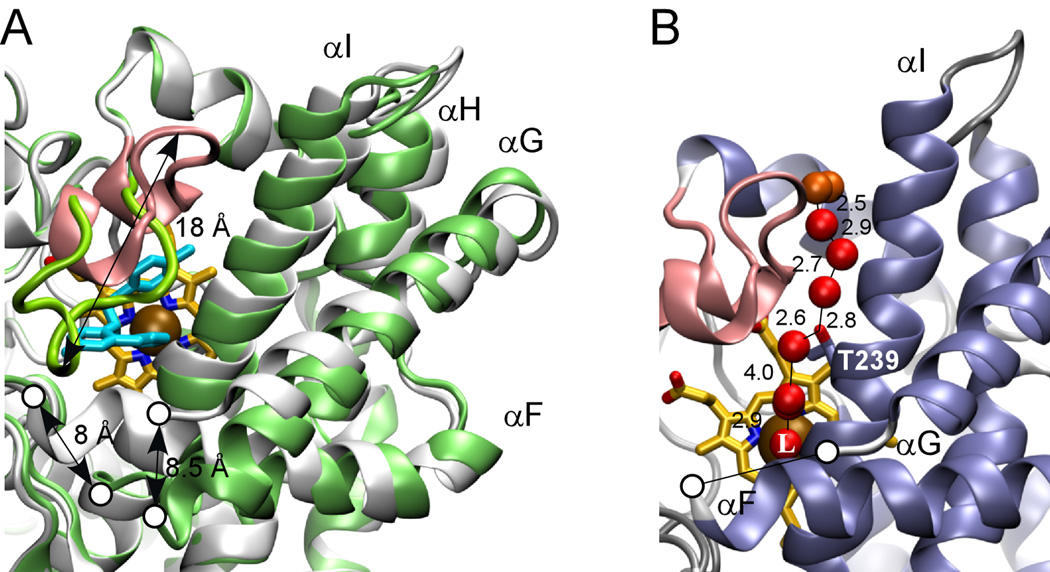
Fig. 4. CYP130 active site.
A, major conformational differences between the ligand-free (gray) and econazole–bound (green) states. The BC-region is in pink, heme is in yellow and econazole is in cyan. Gaps in the protein chain between the F and G helices due to the missing electron density are marked with the open circles. Relocation distances for selected structural elements are given in Angstroms. B, H-bonding network. The fragment of the ligand-free crystal structure showing the water molecules (red spheres) that stabilize the distal water in CYP130. Water molecules having contacts with the bulk solvent are colored in orange. Distances between oxygen atom centers are in Angstroms. T239 is shown in sticks. The iron axial water ligand is labeled with a capital L.