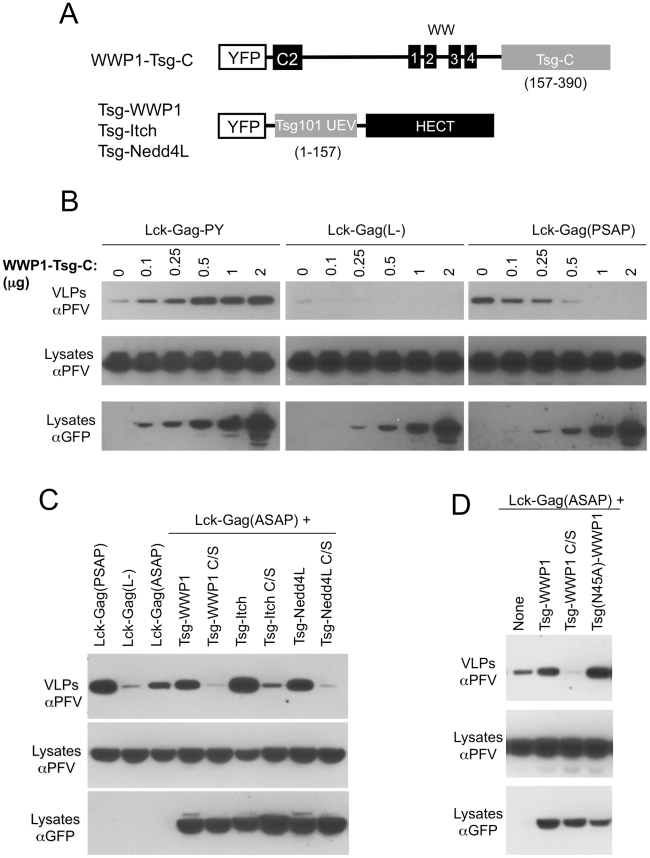
Figure 4. Exchange of functional domains in L-domain cofactors.
(A) Schematic representation of chimeric proteins designed to recruit ESCRT-I to PPxY motif (WWP1-Tsg-C), and isolated catalytic domain of a HECT ubiquitin ligase to a PTAP motif (Tsg-WWP1, Tsg-Itch and Tsg-Nedd4L). (B) Stimulation of PPxY-dependent budding in the absence of a HECT domain by direct recruitment of ESCRT-I. Specifically, VLP production from 293T cells expressing Lck-Gag-PY, Lck-Gag(L-) or Lck-Gag(PSAP) and increasing amounts of YFP-fused WWP1-Tsg-C (the amount of cotransfected WWP1-Tsg-C expression plasmid (in µg) is indicated) was assessed by western blotting. (C,D) VLP production from 293T cells expressing Lck-Gag containing either a wild-type (Lck-Gag(PSAP)), inactive Lck-Gag(L-), or attenuated Lck-Gag(ASAP) late domain and the indicated WT or catalytically inactive mutant (C/S) chimeric Tsg-WWP1, Tsg-Itch and Tsg-Nedd4L proteins was assessed by western blotting. The three leftmost lanes in (C) and the single leftmost lane in (D) contain lysates from cells transfected with unfused YFP as a control in place of a YFP-fused Tsg-HECT protein. Note that the unfused YFP is not visible in these lanes because it migrates to a different position on the blot.