Abstract
Fear can be acquired vicariously through social observation of others suffering from aversive stimuli. We found that mice (observers) developed freezing behavior by observing other mice (demonstrators) receive repetitive foot shocks. Observers had higher fear responses when demonstrators were socially related to themselves, such as siblings or mating partners. Inactivation of anterior cingulate cortex (ACC) and parafascicular or mediodorsal thalamic nuclei, which comprise the medial pain system representing pain affection, substantially impaired this observational fear learning, whereas inactivation of sensory thalamic nuclei had no effect. The ACC neuronal activities were increased and synchronized with those of the lateral amygdala at theta rhythm frequency during this learning. Furthermore, an ACC-limited deletion of Cav1.2 Ca2+ channels in mice impaired observational fear learning and reduced behavioral pain responses. These results demonstrate the functional involvement of the affective pain system and Cav1.2 channels of the ACC in observational social fear.
Fear is a biological response to dangerous, threatening situations or stimuli. Fear can be acquired and expressed in a variety of ways1. First, fear can be learned from direct experience of an adverse situation (for example, an unconditioned stimulus in classical Pavlovian fear conditioning). In a classical conditioning experiment, pairing of a neutral, conditioned stimulus (for example, a tone) with an aversive, unconditioned stimulus (for example, a foot shock) causes an animal to express fear behaviors when the animal is later exposed to the conditioned stimulus in the absence of the unconditioned stimulus. The neural mechanism and circuitry of this fear has been well studied across species, including rodents2,3. Fear can be socially acquired from instruction by verbal information (instructed fear) or from a vicarious observation of a conspecific’s distress (observational fear) in primates, including humans1,4,5. For example, a higher primate can recognize fear by observing a conspecific’s distressed face or a conspecific suffering from an enemy attack1,6–12. Previous studies using a bar-pressing protocol found that rats seeing a distressed conspecific (by electric shocks) display fearful behavioral responses, such as crouching or motionlessness13,14. A recent study found that C57BL/6J mice that observed unfamiliar mice experiencing classical fear conditioning displayed freezing behaviors when they were later exposed to the conditioned stimulus alone15. These findings demonstrate social transfer of fear in rodents. Unlike classical fear conditioning, however, the neural substrate and mechanism underlying observational social fear has not been well defined.
ACC is known to receive sensory signals from the somatosensory cortices and other cortical areas, including the anterior insular cortex16–20. Brain-imaging studies in humans have shown that the neuronal activities of the ACC and the amygdala change during observation of others experiencing fear or others’ fearful facial expressions6,8,10,11. In addition, animal studies have suggested that the ACC is involved in pain affection or emotion behavior, as well as pain sensation21–23. Thus, the ACC is considered to be an important brain region for the convergence of sensory and emotional information and may mediate affective or emotional responses to noxious stimuli. However, the functional involvement of the ACC in observational social fear learning in animals remains unknown.
Brain-imaging studies in humans have shown that some brain regions related to the affective or emotional dimension of pain are activated during observation of distress in individuals suffering from noxious stimuli20,24–26. There are at least two pathways of pain processing via the thalamus in the CNS16,17,26,27. One is the lateral pain system, which expresses the sensory or discriminative dimension of pain and involves the perception of intensity, location and quality of pain stimuli. This pathway projects from the spinal cord dorsal horn to the ventral posterolateral (VPL) and posteromedial (VPM) thalamic nuclei, which relay nociceptive information to the somatosensory cortices. The other is the medial pain system, which represents the affective or emotional dimension of pain and involves perception of the unpleasantness of pain. In this pathway, the spinal nociceptive inputs project to the midline and intralaminar thalamic nuclei (MITN), including parafascicular and mediodorsal thalamic nuclei, and then proceed to limbic cortical areas, including the ACC.
We hypothesized that the medial pain system, such as the ACC, representing affective or emotional components of pain might contribute to social learning of fear by observation. From previous studies13,14, we developed an assay system to evaluate observational fear conditioning in the mouse and confirmed our hypothesis. In addition, we found that the Cav1.2 (α1C, Cacna1c) subunit of the L-type Ca2+ channel, which is known to contribute to synaptic transmission and neuronal excitability28–31, in the ACC could be required in social fear learning.
RESULTS
Observational fear conditioning in the mouse
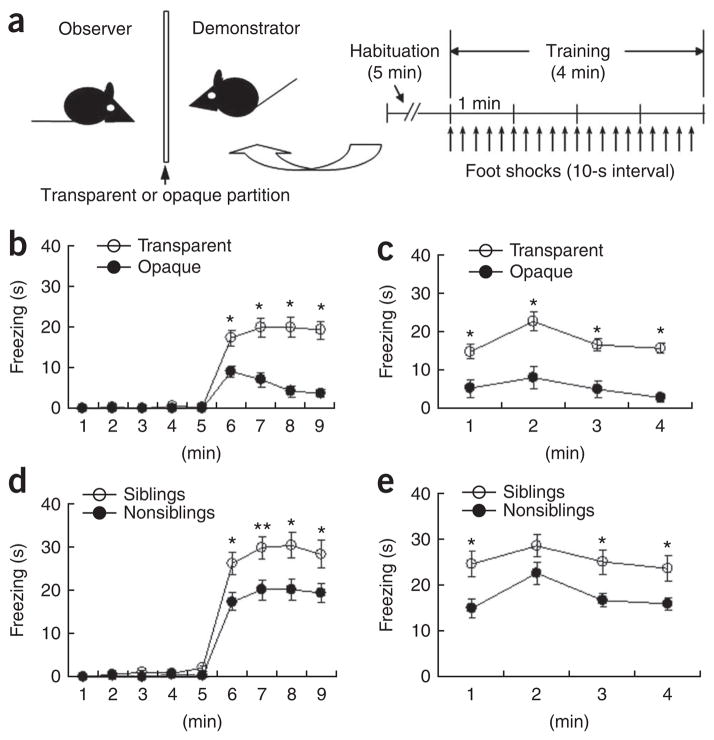
We developed an observational fear conditioning system to study social fear learning in the mouse. In this conditioning system, two male C57BL/6J mice were singly placed in each chamber of a double-chambered fear-conditioning apparatus separated by a transparent Plexiglas partition and one mouse (observer) was allowed to observe the other (demonstrator) as it was subjected to repetitive foot shocks in the opposite chamber (Fig. 1a; control experiments are described in Supplementary Discussion and Supplementary Fig. 1). The observer clearly showed freezing behavior as he saw the demonstrator suffering from foot shocks (Fig. 1b). When the observers alone were placed back into the same observing chamber 24 h after the training, they displayed freezing behaviors (Fig. 1c). However, when the observers were placed into a novel chamber 24 h after the training, they did not exhibit freezing behaviors (Supplementary Fig. 1), indicating that the conditioning was context specific32. These results indicate that observers learned, or were conditioned for, fear that was associated with the context. When the visual inputs were blocked by replacing the transparent partition with an opaque black panel, the fear response of the observers was significantly reduced (F1,27= 30.46, P < 0.0001, two-way repeated ANOVA; Fig. 1b) under the otherwise similar situations and the 24-h contextual fear memory was also reduced (F1,27= 34.30, P < 0.0001, two-way repeated ANOVA; Fig. 1c). Nevertheless, there was still residual freezing behavior when an opaque black partition prevented the observers from seeing the demonstrators (Fig. 1b,c). This indicates that other sensory modalities, such as olfactory and auditory cues, also contribute to the development of the conditioning. Taken together, these results indicate that mice can become conditioned to, or learn, fear by observing the behavior of a conspecific demonstrator.
Figure 1.
Observational fear learning in the mouse. (a) Diagram of the apparatus used for observational fear conditioning and the scheme of the behavioral assay. (b,c) Observational fear learning in the mouse (nonsiblings) using a transparent (n = 21) or opaque (n = 8) partition. A significant difference in the level of freezing behavior was apparent depending on whether a transparent or an opaque partition was used for the conditioning experiment on both the training day (b) and 24 h after training (c). *P < 0.01, Scheffe’s post hoc test. (d,e) Observational fear learning with siblings. We examined freezing behavior on the day of training (F1, 45 = 9.41, P = 0.0036, two-way repeated ANOVA, d) and 24 h after training (F1, 45 = 11.48, P = 0.0015, two-way repeated ANOVA, e) in siblings (n = 26) and nonsiblings (n = 21) using a transparent partition. *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
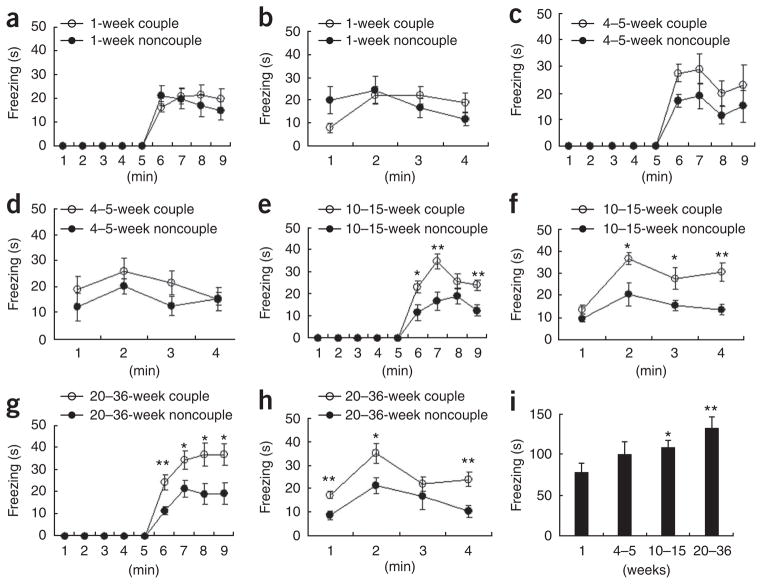
To examine whether the relatedness of the demonstrator to the observer affects observational fear conditioning, we used siblings or female mating partners as demonstrators in the conditioning experiments. Notably, observers exhibited more freezing behavior during both conditioning (Fig. 1d) and the 24-h contextual memory test (Fig. 1e) when the demonstrators were siblings than they did when the demonstrators were unrelated. Next, we used female mating partners as demonstrators (couple and noncouple experiments) and tested whether the length of time that the couple was housed together affects the observational fear learning behavior of the observer male mice (Fig. 2). We did not observe any substantial differences in freezing in the observer mice during observational conditioning between couple and noncouple experiments after housing the mice together for 1 week (Fig. 2a,b) or 4–5 weeks (Fig. 2c,d). However, when female mating partners who lived together more than 10 weeks (10–15-week period and 20–36-week period; Fig. 2e–h) were used as demonstrators (couple experiments), male observers showed more freezing behaviors than was the case when unrelated female mice were demonstrators (noncouple experiments) during the training and the 24-h contextual memory test. Notably, the housing duration showed a graded effect on the development of observational fear (Fig. 2i). These results indicate that familiarity is a vital factor (fear was greater when the demonstrator was more familiar to the observer) in observational fear conditioning. The number of fecal droppings, an indirect measure of fear33, also increased when the demonstrator was related to the observer (Supplementary Fig. 2). Taken together, these findings indicate that the magnitude of the fear response is dependent on the relatedness or familiarity of the demonstrator to the observer and suggest that social relationship is an important element in observational fear conditioning in the mouse.
Figure 2.
Observational fear learning with female mating partners as demonstrators: effect of the duration of co-housing period (familiarity). (a,b) Observational fear conditioning after 1 week of co-housing (couple, n = 10; noncouple, n = 10). There was no difference in the observational fear response (a) and the 24-h contextual memory (b) between couple and noncouple experiments. (c,d) Observational fear conditioning after a 4–5-week co-housing period (couple, n = 6; noncouple, n = 9). There was no difference in the observational training (c) and the 24-h contextual memory (d) between couple and noncouple experiments. (e,f) Observational fear conditioning after 10–15 weeks of co-housing (couple, n = 12; noncouple, n = 7). There were significant differences in the observational training (F1,17 = 11.41, P = 0.0036, two-way repeated ANOVA, e) and the 24-h contextual memory (F1,17 = 11.77, P = 0.0032, two-way repeated ANOVA, f) between couple and noncouple experiments. (g,h) Observational fear conditioning after 20–36 weeks of co-housing (couple, n = 9; noncouple, n = 7). There were significant differences in the observational training (F1,14 = 8.62, P = 0.0109, two-way repeated ANOVA, g) and the 24-h contextual memory (F1,14 = 17.21, P = 0.001, two-way repeated ANOVA, h) between couple and noncouple experiments. (i) The strength of the fear response was increased with as the duration of the co-housing periods increased. ANOVA (F3,33 = 3.38, P = 0.029) of the total freezing time revealed a graded effect of the duration of co-housing period on the development of observational fear and there was a significant difference in total freezing time between 1-week co-housing period group and 10–15-week or 20–36-week groups. *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
Involvement of ACC and MITN in observational fear learning
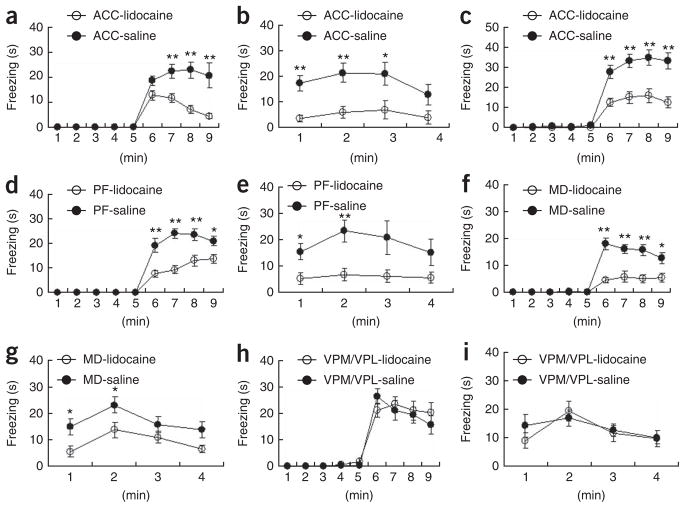
Using this observational fear learning system, we investigated the neuronal substrate or mechanism underlying social fear learning. First, we examined the involvement of the ACC, which is thought to be a cortical substrate for the convergence of sensory and emotional information and may mediate affective or emotional responses to noxious stimuli. For local inactivation, we injected lidocaine (4%) through a cannula into the ACC of the observer 8 min before observational fear training. Lidocaine-injected observers exhibited impaired observational fear learning compared with control mice that were similarly treated with saline (Fig. 3a,b), indicating that the ACC is involved in observational fear learning. Administration of lidocaine to the ACC also impaired observational fear conditioning of mice observing siblings and female mating partners as demonstrators (Fig. 3c). In this experiment, similar freezing levels were obtained when siblings or mating partners were used as demonstrators and the results were thus pooled for analysis: siblings (n = 12) versus couples (n = 7) (F1, 17= 0.38, P = 0.55, two-way repeated ANOVA) with saline and siblings (n = 7) versus couples (n = 6) (F1, 11= 0.20, P = 0.67, two-way repeated ANOVA) with lidocaine (Fig. 3c). We then examined the involvement of parafascicular and mediodorsal thalamic nuclei, which are representative nuclei of MITN projecting to the ACC. When lidocaine was injected into the parafascicular (Fig. 3d,e) or mediodorsal thalamic nuclei (Fig. 3f,g), observers showed impaired observational fear learning compared with control mice that were similarly treated with saline. The impairment was also observed in the 24-h contextual memory test (parafascicular; Fig. 3e) or (mediodorsal; Fig. 3g). These results indicate that the parafascicular and mediodorsal thalamic nuclei are involved in observational fear learning.
Figure 3.
The ACC and MITN are involved in observational fear learning. (a) Mice with lidocaine injections into the ACC (n = 12) before training failed to acquire fear compared with those receiving saline injections (n = 11) (F1, 21 = 19.20, P = 0.0003, two-way repeated ANOVA).
(b) Contextual memory 24 h after the training in a (F1, 21 = 16.43, P = 0.0006, two-way repeated ANOVA). (c) Mice with lidocaine injections into the ACC did not efficiently acquire fear by observation of siblings and mating partners (couples; F1, 30 = 18.25, P = 0.0002, two-way repeated ANOVA). (d,e) Administration of lidocaine into the parafascicular (PF) thalamic nuclei (n = 8) before training led to impaired observational fear learning during training (F1, 15 = 43.84, P < 0.0001, two-way repeated ANOVA, d) and 24 h after training (F1, 15 = 8.55, P = 0.0105, two-way repeated ANOVA, e) as compared with those receiving saline injections (n = 9). (f,g) Administration of lidocaine into the mediodorsal (MD) thalamic nuclei (n = 12) before training caused impaired observational fear learning during training (F1, 28 = 24.11, P < 0.0001, two-way repeated ANOVA, f) and 24 h after training (F1, 28 = 5.19, P = 0.0306, two-way repeated ANOVA, g) as compared with those receiving saline injections (n = 18). (h,i) Administration of lidocaine into the VPL/VPM before training had no influence on the acquisition of observational fear (h) and 24-h contextual memory (i) (lidocaine, n = 10; saline, n = 12). *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
In contrast, a similar inactivation of the VPL/VPM thalamic nuclei, which belong to the lateral, sensory pain system, did not affect observational fear learning (Fig. 3h,i), indicating that these VPL/VPM thalamic nuclei may not be important for the observational fear learning. Taken together, these findings indicate that the ACC, parafascicular and mediodorsal nuclei, but not VPL/VPM nuclei, are involved in observational fear learning and that fear learning requires the medial pain system representing the affective or emotional dimension of pain, but not the lateral system for sensory dimension of pain.
Differential roles of ACC and amygdala in fear conditioning
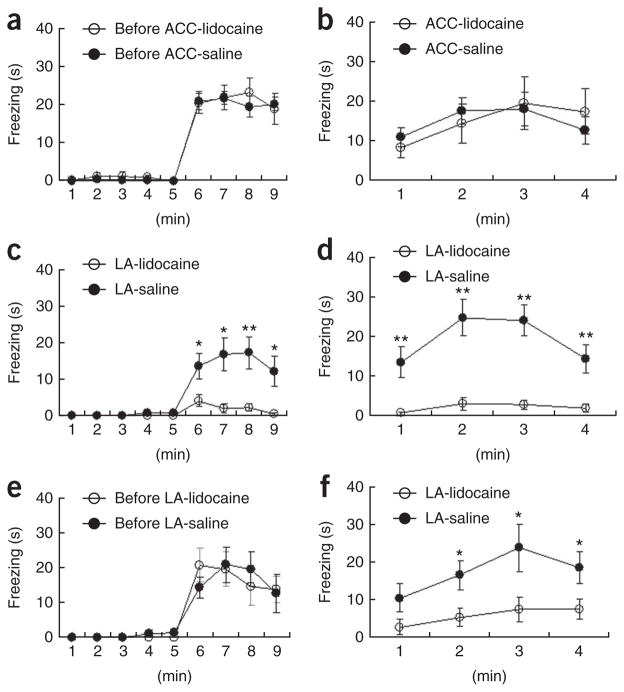
To further study the role of the ACC in long-term memory of observational fear, we subjected mice to observational fear conditioning (Fig. 4a) and then microinjected lidocaine into the ACC 8 min before the 24-h contextual memory test (Fig. 4b). No difference in the freezing level was found between the lidocaine and the control groups (Fig. 4b). The amygdala is believed to be responsible for behavioral reactions to emotional changes, such as noxious stimuli or situations causing aversive or unpleasant consequences2,3. Many studies have found that the amygdala is involved in the recognition of fear or anxiety by observing faces bearing fearful expressions6,8–11. In addition, excitatory anatomical connections have been known to exist between the ACC and the amygdala, including lateral nucleus34. These raise the possibility that there is interaction between the lateral amygdala and the ACC during social fear learning. To test this possibility, we inactivated the lateral amygdala, a brain area that is important for fear learning and memory storage, before the training (Fig. 4c,d) or 24-h contextual memory test (Fig. 4e,f). Inactivation of the lateral amygdala led to disruption of both the acquisition of observational fear (Fig. 4c) and the expression of this fear memory 24 h later (Fig. 4f). These findings are different from the results of ACC inactivation, where the acquisition of observational fear, but not the 24-h memory retrieval or recall was impaired, and thus suggest that the long-term storage of this observational fear memory is not in the ACC, but is instead elsewhere, perhaps in the lateral amygdala.
Figure 4.
The ACC is involved in the acquisition of observational fear, but not in memory retrieval of observational fear and in classical fear conditioning. (a) Mice (n = 9) were trained with observational fear learning. (b) Local inactivation of the ACC 8 min before the 24-h contextual memory test did not affect the expression of fear in observational fear–conditioned mice as compared with fear expression by saline-injected mice (n = 14) (F1, 21 = 0.001, P = 0.99, two-way repeated ANOVA). (c,d) The contribution of the lateral amygdala (LA) to observational fear conditioning. Mice with lidocaine injections into the ACC (n = 8) before training failed to acquire fear compared with those receiving saline injections (n = 10) (F1, 16 = 11.46, P = 0.004, two-way repeated ANOVA, c); the same was true for contextual memory 24 h after training (F1, 16 = 21.34, P = 0.0003, two-way repeated ANOVA, d). (e,f) Local inactivation of the lateral amygdala (n = 8) before the 24-h contextual memory test (f) disrupted the expression of fear in observational fear-conditioned mice (e), as compared with fear shown by saline-injected mice (n = 7) (F1, 13 = 7.66, P = 0.016, two-way repeated ANOVA). *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
We further investigated the role of the ACC in observational fear conditioning and classical fear conditioning. In contrast with its effect on observational fear conditioning, inactivation of the ACC before classical fear conditioning did not affect the mice’s fear response during the training (Supplementary Fig. 3), in 24-h context-dependent memory (Supplementary Fig. 3) or in the 24-h cued memory (Supplementary Fig. 3). This result suggests that ACC is involved in vicarious fear learning by observation, but not in classical conditioning that relies on direct experience of the noxious stimuli of foot shocks, and also strengthens the idea that the ACC is generally associated with the emotional or affective aspects of noxious stimuli.
Synchronized theta activity in ACC and lateral amygdala
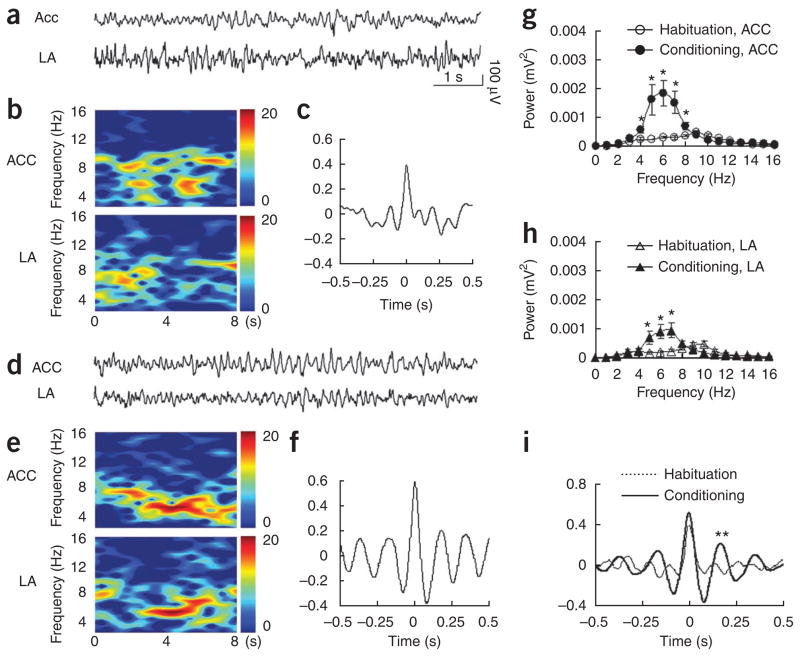
To confirm the functional connectivity of the ACC and the lateral amygdala, we simultaneously measured electrical activities of the ACC and the lateral amygdala by recording local field potentials in freely behaving observers (Fig. 5). We compared the local field potentials before (habituation; Fig. 5a–c) and during observational fear learning (conditioning; Fig. 5d–f). Colored power spectra analysis revealed that the moderate and dispersed neuronal activities in the ACC and lateral amygdala during habituation (Fig. 5b) became intensive and concentrated activities at theta frequency during observational fear learning (Fig. 5e). Theta rhythms became substantially stronger in both the ACC (Fig. 5g) and the lateral amygdala (Fig. 5h) during observational fear learning than they were before the learning. More importantly, nonsynchronized theta activities before training (Fig. 5c) became synchronized theta oscillations between the ACC and the lateral amygdala (Fig. 5f).
Figure 5.
Synchronized theta activity between the ACC and lateral amygdala during learning of fear by observation. (a) Representative original traces of field potential recordings (8 s) in the ACC (upper) and lateral amygdala (bottom) during habituation. (b) Colored power spectra of the traces shown in a. (c) Cross-correlation analysis revealed no correlated neuronal activity in the two brain areas. (d) Representative original traces of field potential recordings in the ACC (upper) and lateral amygdala (bottom) during training. (e) Colored power spectra of the traces shown in d. Note the increased theta rhythms at 4–7 Hz. (f) Cross-correlation analysis revealed correlated neuronal activities in the two brain areas. (g,h) Averaged power spectra of neuronal activities (n = 7) in the ACC (g) and lateral amygdala (h) taken over an 8-s period just before delivery of the first foot shock (habituation) and after the last foot shock (conditioning). *P < 0.05, one-way ANOVA. (i) Averaged cross-correlograms of neuronal activities in the ACC and lateral amygdala taken over an 8-s period just before delivery of the first foot shock (habituation) and after the last foot shock (conditioning) (n = 7). ** indicates a significant difference in the amplitude of the second peaks between the two (P < 0.05, Student’s t test).
Thus, a cross-correlation analysis35 revealed significant synchronization of theta rhythms (at 5.9 ± 0.19 Hz) between the ACC and the lateral amygdala during fear learning (the second peak value after the last foot shock, 0.217 ± 0.08; before the first foot shock, 0.01 ± 0.03; P < 0.05, Student’s t test) (Fig. 5i). These results suggest that the ACC and the lateral amygdala may closely interact for dynamic information transfer during observational fear learning.
ACC–limited deletion of Cav1.2 on fear and pain behavior
To further examine the ACC in social fear learning, we generated Cacna1c conditional knockout mice using cre-loxP and flp-Frt (Supplementary Fig. 4), as the Cav1.2 Ca2+ channel is highly expressed and is known to contribute to synaptic transmission and neuronal excitability in the ACC28,29. To delete Cav1.2 locally, we injected purified cell-permeable Cre recombinase (cp-Cre)36 into the ACC (Supplementary Fig. 4). The deletion of Cav1.2 in the ACC was confirmed by immunostaining (Supplementary Fig. 4).
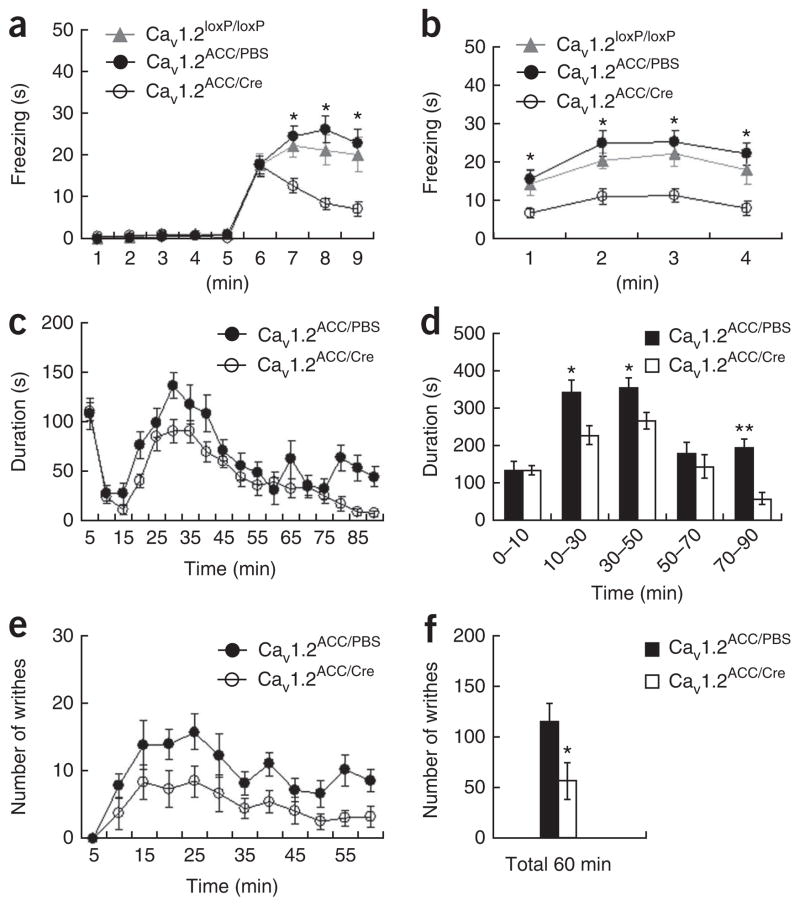
We generated locally deleted Cacna1c (Cav1.2ACC/Cre) mice and used them for various behavioral assays. The Cav1.2ACC/Cre observers exhibited impaired observational fear compared with control observers injected with phosphate-buffered saline (PBS, Cav1.2ACC/PBS) or observers without any treatment (Cacna1cloxP/loxP, referred to as Cav1.2loxP/loxP mice) (Fig. 6a,b). In addition, the Cav1.2ACC/Cre observers shed fewer fecal droppings during observational fear conditioning than the other groups (Supplementary Fig. 2). When an opaque partition was used during observational conditioning, all groups displayed similarly decreased levels of freezing behavior compared to those with a transparent partition (Supplementary Fig. 5). Notably, the Cav1.2ACC/Cre mice displayed reduced pain behavioral responses to formalin (Fig. 6c,d) and acetic acid (Fig. 6e,f), as compared with Cav1.2CC/PBS mice. However, there was no difference between the two groups in acute pain behavior, either mechanical or thermal (Supplementary Fig. 5), which is known to be mediated by a spinal reflex37. These results suggest that functional Cav1.2 type 1 Ca2+ channels in the ACC are required for observational social fear learning and are critical for pain response modulation at the supraspinal levels, further supporting the involvement of the ACC in affective or emotional dimension of noxious or aversive stimuli20,24–26. In contrast, Cav1.2ACC/Cre mice showed normal behavioral responses in elevated plus maze (Supplementary Fig. 6), light/dark transition (Supplementary Fig. 6), open field (Supplementary Fig. 6), novel object recognition (Supplementary Fig. 7), predator exposure (Supplementary Fig. 7) and classical fear conditioning tasks (Supplementary Fig. 7).
Figure 6.
Cav1.2ACC/Cre mice showed impaired observational fear learning and reduced pain responses. (a,b) Observational fear conditioning of Cav1.2ACC/Cre (n = 22), Cav1.2ACC/PBS (n = 22) and Cav1.2loxP/loxP (n = 13) mice. Similar freezing levels were seen during training (F1, 33 = 0.48, P = 0.49, two-way repeated ANOVA) and in the 24-h contextual memory test (F1,33 = 0.95, P = 0.34, two-way repeated ANOVA) between Cav1.2ACC/PBS (PBS injected) and Cav1.2loxP/loxP (non-injected) observers, and the results were pooled for analysis. The Cav1.2ACC/Cre observers exhibited impaired observational fear learning during training (F1, 55 = 17.47, P < 0.0001, two-way repeated ANOVA, a) and 24-h contextual memory (F1, 55 = 20.85, P < 0.0001, b). *P < 0.01, Scheffe’s post hoc test. (c,d) Reduced inflammatory pain responses to formalin in Cav1.2ACC/Cre mice. Behavioral responses to a formalin injection, plotted in 5-min intervals, in Cav1.2ACC/PBS mice (n = 9) compared with Cav1.2ACC/Cre mice (n = 15) are shown in c. Data from c were grouped into five time intervals (d). *P < 0.05, **P < 0.0001, one-way ANOVA.
(e,f) Reduced behavioral responses to acetic acid–induced visceral pain in Cav1.2ACC/Cre mice. Behavioral responses to acetic acid, plotted in 5-min intervals, in Cav1.2ACC/PBS mice (n = 7) and Cav1.2ACC/Cre mice (n = 6) are shown in e. The total numbers of writhing events over 60 min are shown in f. *P < 0.05, one-way ANOVA. Error bars represent s.e.m.
DISCUSSION
We developed and characterized a behavioral assay system for observational fear conditioning in the mouse. Using this behavioral assay, we further investigated the neuronal substrate and mechanism underlying observational social fear learning. We found that the ACC and the MITN (parafascicular and mediodorsal thalamic nuclei), which consist of the medial pain system representing the affective or emotional dimension of pain, are involved in observational fear. In contrast, the VPL/VPM thalamic nuclei that belong to the lateral, sensory pain system may not be involved in the process. Notably, the lateral amygdala, but not the ACC, was involved in the expression of the observational fear memory. Augmented and synchronized theta activities between the ACC and the lateral amygdala were observed during this social fear behavior. In addition, the Cav1.2 type 1 Ca2+ channels in the ACC were required for the observational fear behavior. These data suggest a neural substrate and a cellular or molecular mechanism of social fear learning in the mouse.
Lesion and acute inactivation studies have shown that the ACC is involved in modulating the efficiency of trace auditory fear learning requiring high attention34,38. However, those lesions did not affect the expression of the memory34. In addition, other lesion studies on avoidance learning found that an ACC lesion after training did not impair the expression of conditioned place aversion, indicating that the ACC was involved in producing an aversive teaching signal, but not in memory retrieval for context39,40. Similarly, our results indicate that lateral amygdala inactivation disrupts both the acquisition and the retrieval of observational fear, whereas ACC inactivation only affects acquisition. These results suggest that the lateral amygdala is essential for the learning and memory storage of fear, whereas the ACC may have a modulatory role in the development of fear by integrating sensory and affective components of information.
Inactivation of the MITN decreased observational fear behavior to a similar extent as inactivation of the ACC (Fig. 3). However, inactivation of the parafascicular nuclei had no influence on classical fear conditioning (Supplementary Fig. 8), similar to that of the ACC (Supplementary Fig. 3). On the other hand, an inactivation of VPL/VPM thalamic nuclei, the sensory pain system, had no effect on social fear learning by observation, although the same inactivation decreased the pain response behavior of the mice (Supplementary Fig. 8). These results indicate that the medial pain system, but not the lateral sensory pain system, is involved in observational social fear learning. At the moment, however, we cannot rule out nonserial involvements of the ACC and the MITN. In fact, there was a difference in the freezing levels among the observers with lidocaine injection into different brain areas (ACC, parafascicular, mediodorsal and lateral amygdala) before training (Supplementary Fig. 8). The interpretation of these results, however, is limited by the lack of evidence for a complete inactivation of each nucleus. Further studies with crossed inactivation or disconnection of the ACC or lateral amygdala and the MITN will provide valuable information regarding whether these brain areas are involved in observational fear learning in serial processing.
It has been shown that synchronization of neuronal activities is a potential mechanism linking anatomically and functionally related regions of the brain35,41,42. The amygdala is widely believed to be responsible for behavioral reactions to emotional changes, such as noxious stimuli or situations causing aversive or unpleasant consequences, and previous work using images of facial expression has shown that activation of the amygdala, as well as the ACC, is necessary for fear recognition by observation of others experiencing fear or others’ fearful facial expressions6,8,10,11. Thus, investigation of the neuronal activity between the ACC and the amygdala can provide important information on observational fear learning. The ACC is also known to be involved in executive processes, attentional processes and decision making, and a number of electroencephalography studies have shown that theta (4–7 Hz) oscillations around the ACC and prefrontal cortex occur in a variety of behavioral tasks, particularly in tasks involving working memory and mental efforts in humans18,43–45. Our results suggest that theta oscillations are also involved in observational fear recognition and that the synchronized activity in the ACC– lateral amygdala occurs during this behavior. Therefore, the increased coherent theta activities in the ACC–lateral amygdala may represent and promote neuronal communication required for recognition and expression of social fear.
We generated a mouse model for studying impaired social fear learning, a mouse with an ACC-limited deletion of Cav1.2 Ca2+ channels. The Cav1.2ACC/Cre mice showed not only disrupted social fear learning by observation, but also reduced pain responses. Notably, the Cav1.2ACC/Cre mice showed a level of anxiety (Supplementary Fig. 6) and innate fear (Supplementary Fig. 7) that was similar to that of control mice. Thus, these results suggest that the neural mechanisms underlying observational social fear behavior may be different from those for anxiety or innate fear. In addition, the Cav1.2ACC/Cre mice showed normal performance in the object recognition task with inanimate objects (Supplementary Fig. 7), suggesting that the processing of individual information obtained by observing different objects varies depending on the objects (a conspecific, a live predator and an inanimate object). The Cav1.2ACC/Cre mice had a normal fear response in classical fear conditioning (Supplementary Fig. 7), as did the ACC-inactivated mice (Supplementary Fig. 3). At this point, we do not know the role of Cav1.2 in observational fear. One possibility is that the effects of the Cav1.2 deletion might be the result of a general decrease of excitability in the ACC. Further electrophysiological or pharmacological experiments will reveal the role of Cav1.2 in this process. To the best of our knowledge, this is the first animal behavioral study to use the cp-Cre recombinase in the cre-loxP site-specific recombination system in vivo, suggesting that direct application of cp-Cre can be a useful tool for studying the time- and tissue-specific role of a gene of interest.
The observers showed higher levels of fear responses when siblings or female mating partners were used as the demonstrators than when the demonstrators were not siblings or related female mice. These results suggest that something more than just emotional contagion could be involved in observational social fear learning. Particularly, the strength of the fear response was increased with increasing familiarity of the observer to the demonstrator (couples experiments). Familiarity is considered to be a factor in making the observer more empathetic for the situation or state of the demonstrator in primates46. Therefore, if empathy is broadly used as a term representing affective behaviors focused on the response of the observer47, empathy might be engaged in observational social fear learning, as in the case of the social modulation of pain48. However, although there were apparently no aggressive behaviors such as dashing toward the other mouse during the training, the responses of the demonstrators to repetitive shocks might be experienced as a dangerous (threatening) or stressful action by the observers, rather than the observers having an empathic fear response for the demonstrators. Future studies are necessary to clarify this point.
In our observational fear assay, it appears that the sensory modalities (olfactory, auditory and visual cues) could have a synergistic effect on the observers. This is supported by the results of the experiments in which an opaque partition selectively removed the visual input. Even under this condition, siblings or couples induced higher freezing levels in observers than nonsiblings or noncouples, respectively (Supplementary Fig. 9).
Here we developed a behavioral assay system to measure social fear conditioning by observation in the mouse and found that mice can learn fear without first-hand experience of noxious stimuli (for example, foot shocks). We found that the affective pain system is involved in social fear learning by observation and identified the Cav1.2 Ca2+ channel in the ACC as an essential element in the process. Many aberrant social behaviors associated with psychiatric conditions, including various psychopathic or mental disorders (for example, post-traumatic stress disorders, schizophrenia, autism and dementia), feature impairment of recognition of the emotions and feelings of others and dysfunctions in the ACC have been associated with these psychiatric conditions19,49. We suggest that this behavioral model for social fear can be a useful tool for developing measures to control such disorders.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Acknowledgments
We thank K. Lee for help with Matlab analysis. This work was supported by the National Honor Scientist program of Korea and the Center of Excellence program from the Korea Institute of Science and Technology.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
D. Jeon and H.-S.S. designed the experiments. D. Jeon purified Cre protein. D. Jo and H.E.R. made and provided the vector containing His6-NLS-Cre-MTS. D. Jeon, S.K. and M.C. performed surgeries, microinjections and immunostainings and analyzed the data. D. Jeon and S.K. performed in vivo electrophysiology. S.-Y.L., D.R. and J.-P. K. generated the Cav1.2 conditional mice. D. Jeon and H.-S.S. wrote the manuscript. All of the authors commented on the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprintsandpermissions/.
References
- 1.Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Adolphs R. Cognitive neuroscience of human social behavior. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 5.Frith CD. Social cognition. Phil Trans R Soc Lond B. 2008;363:2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooker CI, Germine LT, Knight RT, D’Esposito M. Amygdala response to facial expressions reflects emotional learning. J Neurosci. 2006;26:8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. J Exp Psychol Gen. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson A, Phelps EA. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychol Sci. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- 10.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 11.Whalen PJ, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RE, Murphy JV, Mirsky IA. Non-verbal communication of affect. J Clin Psychol. 1959;15:155–158. doi: 10.1002/1097-4679(195904)15:2<155::aid-jclp2270150211>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Rice GE, Gainer P. “Altruism” in the albino rat. J Comp Physiol Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 14.Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–134. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 17.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 20.Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 21.Gao YJ, Ren WH, Zhang YQ, Zhao ZQ. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain. 2004;110:343–353. doi: 10.1016/j.pain.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaGraize SC, et al. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol. 2004;188:139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 26.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auvray M, Myin E, Spence C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci Biobehav Rev. 2010;34:214–223. doi: 10.1016/j.neubiorev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Day M, et al. Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Cav1.2 L-type Ca2+ currents via a PLCβ/IP3/calcineurin signaling cascade. J Neurophysiol. 2002;87:2490–2504. doi: 10.1152/jn.00843.2001. [DOI] [PubMed] [Google Scholar]
- 29.Liauw J, Wu LJ, Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol. 2005;94:878–882. doi: 10.1152/jn.01205.2004. [DOI] [PubMed] [Google Scholar]
- 30.Meredith RM, et al. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Moosmang S, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor–independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14:313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bissière S, et al. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 36.Jo D, et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nat Biotechnol. 2001;19:929–933. doi: 10.1038/nbt1001-929. [DOI] [PubMed] [Google Scholar]
- 37.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 38.Han CJ, et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci USA. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 40.Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci USA. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 42.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 43.Raghavachari S, et al. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- 44.Sarnthein J, et al. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuhara H, Yamaguchi Y. Human cortical circuits for central executive function emerge by theta phase synchronization. Neuroimage. 2007;36:232–244. doi: 10.1016/j.neuroimage.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman ML. Empathy, its development and prosocial implications. Nebr Symp Motiv. 1977;25:169–217. [PubMed] [Google Scholar]
- 48.Langford DJ, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 49.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II. Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.