Figure 3. Asp3 forms complexes with Asp1, Asp2, Asp3 and SecA2.
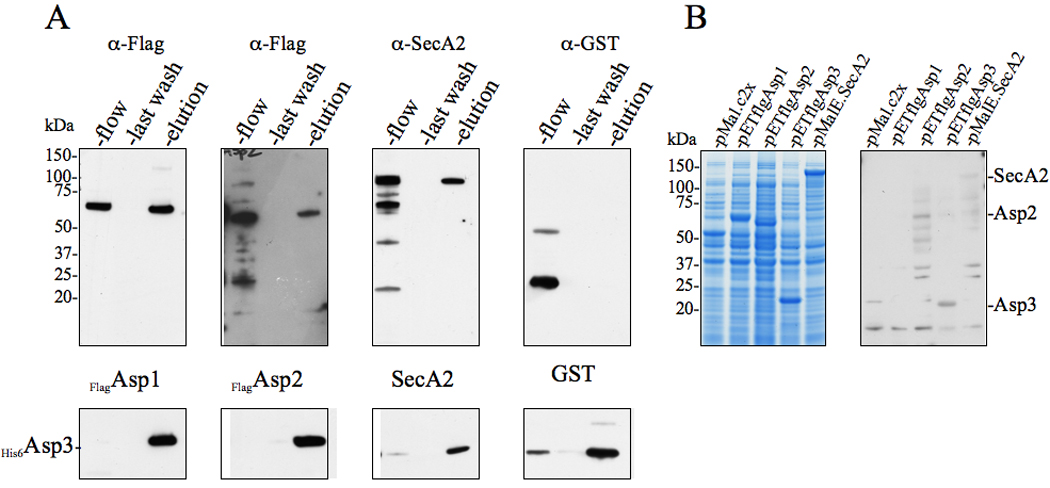
(A) Lysates of E. coli BL21 (DE3) co-expressing His6Asp3 and Flag-tagged Asp1, Asp2, or SecA2 were applied to Ni2+ agarose, followed by washing of the columns and elution of the retained material. Lysates of cells co-expressing His6Asp3 and GST served as negative controls for nonspecific binding. The various fractions (flow, last wash, elution) were probed by Western blotting for proteins co-purifying with His6Asp3. Eluted His6Asp3 was detected using anti-His6 antibody (lower panel) and co-purifying proteins were detected with anti-Flag, anti-SecA2 or anti-GST antibodies (upper panel).
(B) E. coli BL21 (DE3) lysates expressing MalE, flagAsp1, flagAsp2 flagAsp3 or MalE.SecA2 were electrophoresed in SDS-PAGE (4–12%) and either stained with Coomassie blue (left panel), or transferred to membranes and probed with His6Asp3 (right panel). Binding of Asp3 to the immobilized proteins was detected with anti-His6 antibody.
