Figure 4. Effects of Asp3-Asp1 interaction upon the export of GspB736flag .
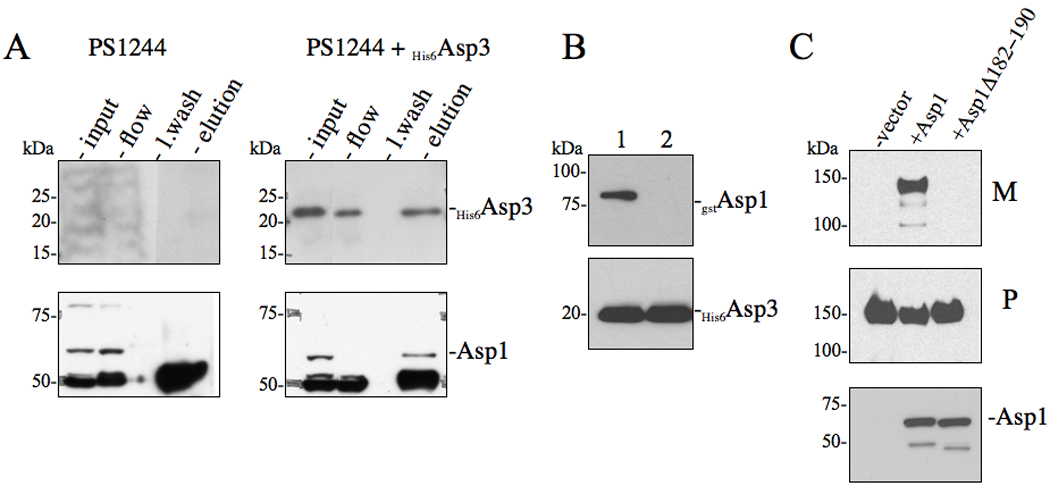
(A) in vivo co-immunoprecipitation of Asp3 with accessory Sec components. S. gordonii PS1244 was complemented in trans with His6Asp3 or vector alone. Clarified lysates were applied to anti-Asp3 resin, followed by washing of the column and elution of the retained material. The various fractions (flow, last wash, elution) were probed by Western blotting for proteins co-purifying with His6Asp3. Eluted His6Asp3 was detected using anti-His6 antibody (upper panel) and the co-purifying protein was detected with anti-Asp1 antisera. (B) Co-expression co-purification of Asp3-Asp1 complexes. Lysates of E. coli BL21 (DE3) co-expressing His6Asp3 with either (1) gstAsp1 or (2) gstAsp1 (Δ182−190) were applied to Ni2+ agarose, followed by washing of the columns and elution of the retained material. Eluted material was probed by Western blotting for proteins co-purifying with His6Asp3. Eluted His6Asp3 was detected using anti-His6 antibody (lower panel) and co-purifying Asp1 was detected with anti-GST antisera (upper panel). (C) Western blot analysis of GspB736flag export from S. gordonii PS1242 derivative strains carrying internal deletions within Asp1. Culture media (M) and protoplasts (P) were collected from exponentially growing strains and prepared as described in the methods and materials. Proteins were separated by SDS-PAGE (3–8%) and following Western blot transfer, GspB736flag was probed with anti-flag antibodies.
