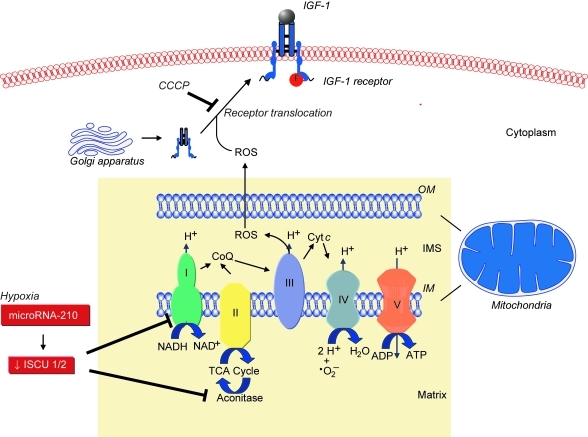
FIG. 1.
Mitochondrial ROS, metabolism, and role in translocation and function of disulfide-containing cell-surface protein receptors. ROS generated by mitochondrial respiration play an integral role in the cell-surface translocation and activation of selected disulfide containing proteins, such as insulin growth factor-1 (IGF-1) receptor. In the presence of mitochondrial ROS inhibitors, such as the mitochondrial uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP), translocation of the IGF-1 receptor from the cytoplasm to the cell membrane is inhibited. Inhibition of mitochondrial ROS also limits phosphorylation and activation of the receptor in CCCP-treated endothelial cells. Under hypoxic conditions, mitochondrial metabolism shifts, leading to a repression of electron transport and oxidative phosphorylation in favor of glycolysis. This phenomenon is known as the “Pasteur effect.” This metabolic switch is regulated by microRNA-210, which is upregulated by hypoxia, and downregulates the expression of iron-sulfur cluster assembly proteins 1 and 2 (ISCU 1/2). These proteins are necessary for the activity of Complex I and aconitase. Thus, microRNA-210 limits mitochondrial electron transport, ROS, and the TCA cycle. CoQ, Coenzyme Q; OM, outer mitochondrial membrane; IM, inner mitochondrial membrane. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).