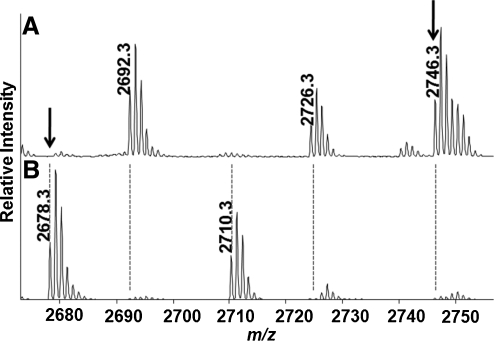
FIG. 3.
Differential alkylation of SIRT1-Flag. Purified mouse SIRT1 was incubated in the presence of 2.0 mM GSNO for 15 min at 37°C. Unreacted thiols were blocked with NEM. Initial alkylation is followed by addition of DTT to remove GSH adducts, and then a second alkylation step with IAM. (A) Thiols that were reactive with GSNO were expected to be labeled by the second alkylating agent following reduction with DTT. A major peak with m/z 2746.313 was identified that corresponds to the peptide R46SPGEPSAAVAPAA AGCEAASAAAPAALWR74 with an additional +125 Da, indicating that the cysteine is alkylated with NEM. (B) Control protein that was not exposed to GSNO was also differentially alkylated and both spectra were compared. In this spectrum a peak was identified with m/z 2678.277 corresponding to the same peptide bearing a carbamidomethylation (+57 Da) rather than modification by NEM. Samples were subjected to SDS-PAGE, in-gel trypsin digestion, and analysis by MALDI-TOF. The converse experiment in which IAM was used initially, followed by NEM labeling of cysteines reduced by DTT, was used to control for varying reactivities of alkylating reagents, and as indicated in Table I showed similar results.