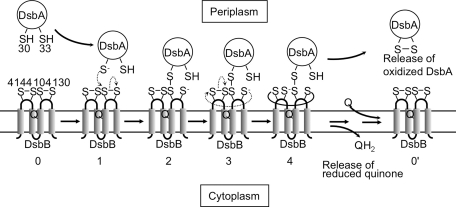
FIG. 4.
A model for early steps in the reoxidation of DsbA by DsbB. DsbA is highly oxidizing. Thus, it tends to be reduced. The mechanism by which DsbB oxidizes such a highly oxidizing protein is a fundamental question. In this model, the interloop disulfide bond formed between Cys130 and Cys41 (stage 4) prevents the back reaction (stage 2 to stage 0) that would tend to occur because of the highly oxidizing nature of DsbA. Thus, DsbB coordinates the action of its four cysteines to oxidize DsbA. In this model, the DsbB–DsbA complex in stage 4 corresponds to the charge-transfer complex that is depicted in Fig. 5. In the alternative model, electron transfer from DsbA to quinone proceeds through a sequential set of two thiol-disulfide exchange reactions, and then reduction of quinones through a charge-transfer complex (not shown). Q indicates the position of ubiquinone bound to DsbB.