Abstract
The title compound, (C6H21N4)[TiF6]F, was synthesized by the reaction of TiO2, tris(2-aminoethyl)amine, HF and ethanol at 463 K in a microwave oven. The crystal structure consists of two crystallographically independent [TiF6]2− anions, two fluoride anions and two triply-protonated tris(2-aminoethyl)amine cations. The Ti atoms are coordinated by six F atoms within slightly distorted octahedra. The anions and cations are connected by intermolecular N—H⋯F hydrogen bonds.
Related literature
For background, see: Adil et al. (2006 ▶). For related structures, see: Calov et al. (1992 ▶); Dadachov et al. (2000 ▶); Tang et al. (2001 ▶).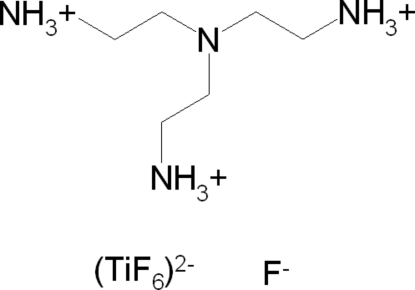
Experimental
Crystal data
(C6H21N4)[TiF6]F
M r = 330.14
Monoclinic,
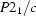
a = 16.265 (4) Å
b = 8.089 (3) Å
c = 21.778 (5) Å
β = 110.54 (2)°
V = 2683.1 (13) Å3
Z = 8
Mo Kα radiation
μ = 0.71 mm−1
T = 298 (2) K
0.18 × 0.13 × 0.06 mm
Data collection
Siemens AED2 diffractometer
Absorption correction: Gaussian (SHELX76; Sheldrick, 2008 ▶) T min = 0.850, T max = 0.929
6191 measured reflections
6133 independent reflections
3531 reflections with I > 2σ(I)
3 standard reflections frequency: 120 min intensity decay: 15%
Refinement
R[F 2 > 2σ(F 2)] = 0.061
wR(F 2) = 0.155
S = 1.12
6133 reflections
332 parameters
H-atom parameters constrained
Δρmax = 1.37 e Å−3
Δρmin = −0.42 e Å−3
Data collection: STADI4 (Stoe & Cie, 1998 ▶); cell refinement: STADI4; data reduction: X-RED (Stoe & Cie, 1998 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2001 ▶); software used to prepare material for publication: enCIFer (Allen et al., 2004 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808009781/nc2095sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808009781/nc2095Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Ti1—F1 | 1.796 (3) |
| Ti1—F2 | 1.826 (3) |
| Ti1—F4 | 1.856 (3) |
| Ti1—F5 | 1.865 (3) |
| Ti1—F3 | 1.868 (3) |
| Ti1—F6 | 1.882 (3) |
| Ti2—F8 | 1.803 (3) |
| Ti2—F7 | 1.821 (3) |
| Ti2—F9 | 1.825 (3) |
| Ti2—F10 | 1.827 (3) |
| Ti2—F11 | 1.832 (3) |
| Ti2—F12 | 1.856 (3) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2C⋯F13 | 0.89 | 1.90 | 2.773 (5) | 165 |
| N2—H2D⋯F6i | 0.89 | 2.04 | 2.865 (5) | 154 |
| N2—H2E⋯F13i | 0.89 | 1.84 | 2.725 (5) | 172 |
| N3—H3C⋯F13 | 0.89 | 1.86 | 2.700 (5) | 157 |
| N3—H3C⋯N1 | 0.89 | 2.52 | 2.948 (6) | 110 |
| N3—H3D⋯F3 | 0.89 | 1.90 | 2.726 (5) | 154 |
| N3—H3E⋯F9 | 0.89 | 1.84 | 2.717 (5) | 167 |
| N4—H4C⋯F13 | 0.89 | 1.83 | 2.692 (5) | 162 |
| N4—H4D⋯F12i | 0.89 | 2.01 | 2.835 (5) | 153 |
| N4—H4E⋯F5 | 0.89 | 1.84 | 2.712 (5) | 168 |
| N6—H6C⋯F14 | 0.89 | 1.84 | 2.696 (5) | 162 |
| N6—H6D⋯F10ii | 0.89 | 2.00 | 2.823 (5) | 154 |
| N6—H6E⋯F4iii | 0.89 | 1.90 | 2.749 (5) | 160 |
| N7—H7C⋯F14 | 0.89 | 1.82 | 2.699 (5) | 169 |
| N7—H7D⋯F2iii | 0.89 | 2.24 | 2.876 (5) | 129 |
| N7—H7E⋯F7i | 0.89 | 2.08 | 2.916 (5) | 157 |
| N7—H7E⋯F10i | 0.89 | 2.41 | 2.972 (5) | 121 |
| N8—H8C⋯F14 | 0.89 | 1.91 | 2.791 (5) | 168 |
| N8—H8D⋯F6 | 0.89 | 2.14 | 2.879 (5) | 140 |
| N8—H8E⋯F14iv | 0.89 | 1.81 | 2.702 (5) | 177 |
Symmetry codes: (i) 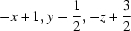 ; (ii)
; (ii) 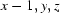 ; (iii)
; (iii) 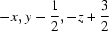 ; (iv)
; (iv) 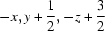 .
.
supplementary crystallographic information
Comment
To date, only a few organic-inorganic fluorotitanates were reported. The first compound {(CN3H6)[TiF6]}, described by Calov et al. (1992), is built up from (TiF6) monomers and guanidinium cations. Dadachov et al. (2000) and Tang et al. (2001) reported the synthesis of piperazinium {(C4N2H12)2[Ti2F10]2(H2O)} and piperidinium {[C5H6N2]2(Ti2F11)(H3O)(H20)} fluorotitanates respectively, built up from (Ti2F10)2- or (Ti2F11)3- dimers. As a part of our ongoing investigations in this field (Adil et al., 2006)) we now report the synthesis and structure of the title compound, (I).
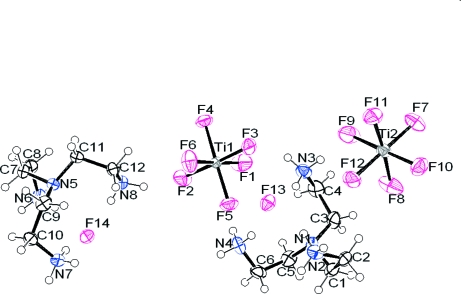
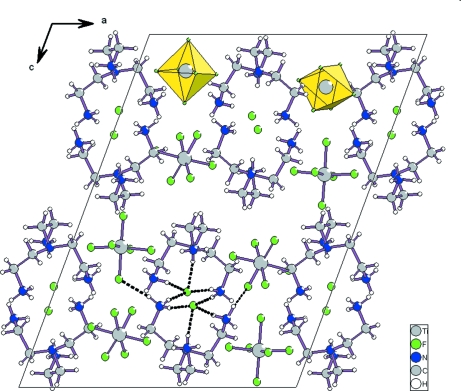
The asymmetric unit of (I) consits of two crystallographically independent (TiF6)2- anions, two fluoride anions and two triprotonated tris(2-aminoethyl)amine (tren) cations, all of them located in general positions (Fig. 1). The TiF6 anions form sligthly distorted octahedra and the environnement of both independent anions is different (Table 1). Both TiF6 anions are connected to the cations via N—H···F hydrogen bonding (Figure 2 and Table 2).
The two isolated fluoride anions are also hydrogen bonded to the [H3tren]3+ cations with N—H···F distances ranging from 2.692 (5)Å to 2.791 (5)Å (Figure 2 and Table 2).
Experimental
The synthetis was performed by using a microwave-assisted route. Crystals were prepared from a mixture of titanium(IV) oxide (79 mg, 1 mmol), tris(2-aminoethyl)amine (0.230 ml, 1.52 mmol), hydrogen fluoride (40%, 0.130 ml, 2.95 mmol) and ethanol (10 ml, 35 mmol). The mixture was transferred into a teflon autoclave installed in a CEM microwave oven at 493 K for 1 hour under a constant pressure of 22 bar. Finally, the solid product was washed with ethanol and dried in air at room temperature to yield colourless paralellepipeds of (I).
Refinement
A number of mis-measured reflections were omitted from the refinement.
The hydrogen atoms were positioned with idealized geometry (C—H = 0.88-0.97Å, N—H = 0.89Å) and modelled as riding with a group Uiso value refined.
The highest difference is 2.49 Å from H4A. It might be that this peak corresponds to a small amount of water, which cannot be proven. Therefore, this electron density was not considered in the final refinement.
Figures
Fig. 1.
Crystal structure of (I) with displacement ellipsoids for the non-hydrogen atoms drawn at the 50% probability level.
Fig. 2.
Crystal packing of (I) with view along [010]. Hydrogen bonding is shown as dashed lines.
Crystal data
| (C6H21N4)[TiF6]F | F(000) = 1360 |
| Mr = 330.14 | Dx = 1.635 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71069 Å |
| Hall symbol: -P 2ybc | Cell parameters from 32 reflections |
| a = 16.265 (4) Å | θ = 29.1–30.9° |
| b = 8.089 (3) Å | µ = 0.71 mm−1 |
| c = 21.778 (5) Å | T = 298 K |
| β = 110.54 (2)° | Parallelepiped, colourless |
| V = 2683.1 (13) Å3 | 0.18 × 0.13 × 0.06 mm |
| Z = 8 |
Data collection
| Siemens AED2 diffractometer | 3531 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.0000 |
| graphite | θmax = 27.5°, θmin = 2.0° |
| 2θ/ω scans | h = −21→19 |
| Absorption correction: gaussian (SHELX76; Sheldrick, 2008) | k = 0→10 |
| Tmin = 0.850, Tmax = 0.929 | l = 0→28 |
| 6191 measured reflections | 3 standard reflections every 120 min |
| 6133 independent reflections | intensity decay: 15% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.061 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.155 | H-atom parameters constrained |
| S = 1.12 | w = 1/[σ2(Fo2) + (0.0532P)2 + 2.5558P] where P = (Fo2 + 2Fc2)/3 |
| 6133 reflections | (Δ/σ)max = 0.001 |
| 332 parameters | Δρmax = 1.37 e Å−3 |
| 0 restraints | Δρmin = −0.42 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ti1 | 0.18135 (5) | 0.59284 (10) | 0.60307 (4) | 0.02406 (18) | |
| Ti2 | 0.70766 (5) | 0.58675 (11) | 0.64839 (4) | 0.0319 (2) | |
| F1 | 0.1489 (3) | 0.5816 (4) | 0.51539 (14) | 0.0687 (10) | |
| F2 | 0.07464 (18) | 0.5254 (4) | 0.60403 (17) | 0.0582 (9) | |
| F3 | 0.29513 (18) | 0.6604 (4) | 0.61299 (17) | 0.0539 (8) | |
| F4 | 0.14665 (19) | 0.8126 (3) | 0.59720 (15) | 0.0469 (7) | |
| F5 | 0.2192 (2) | 0.3737 (3) | 0.61004 (16) | 0.0492 (8) | |
| F6 | 0.2182 (2) | 0.6079 (5) | 0.69502 (13) | 0.0557 (9) | |
| F7 | 0.72742 (19) | 0.7113 (4) | 0.58528 (14) | 0.0518 (8) | |
| F8 | 0.6614 (2) | 0.4174 (4) | 0.59328 (14) | 0.0631 (10) | |
| F9 | 0.5986 (2) | 0.6765 (4) | 0.6291 (2) | 0.0817 (13) | |
| F10 | 0.81774 (18) | 0.4997 (4) | 0.66930 (15) | 0.0494 (8) | |
| F11 | 0.7527 (2) | 0.7485 (4) | 0.70991 (15) | 0.0533 (8) | |
| F12 | 0.6933 (2) | 0.4627 (4) | 0.71561 (15) | 0.0555 (9) | |
| N1 | 0.4459 (2) | 0.1509 (5) | 0.60450 (17) | 0.0276 (8) | |
| C1 | 0.5134 (3) | 0.0224 (6) | 0.6192 (2) | 0.0361 (11) | |
| H1A | 0.5186 | −0.0159 | 0.5785 | 0.044 (2)* | |
| H1B | 0.4952 | −0.0708 | 0.6394 | 0.044 (2)* | |
| C2 | 0.6020 (3) | 0.0814 (6) | 0.6642 (2) | 0.0342 (10) | |
| H2A | 0.6448 | −0.0063 | 0.6703 | 0.044 (2)* | |
| H2B | 0.6206 | 0.1748 | 0.6444 | 0.044 (2)* | |
| N2 | 0.5983 (2) | 0.1311 (5) | 0.72899 (18) | 0.0333 (9) | |
| H2C | 0.5635 | 0.2188 | 0.7239 | 0.044 (2)* | |
| H2D | 0.6521 | 0.1565 | 0.7560 | 0.044 (2)* | |
| H2E | 0.5773 | 0.0479 | 0.7458 | 0.044 (2)* | |
| C3 | 0.4511 (3) | 0.2578 (6) | 0.5509 (2) | 0.0392 (12) | |
| H3A | 0.4246 | 0.2012 | 0.5093 | 0.044 (2)* | |
| H3B | 0.5123 | 0.2784 | 0.5571 | 0.044 (2)* | |
| C4 | 0.4048 (3) | 0.4209 (7) | 0.5489 (2) | 0.0418 (12) | |
| H4A | 0.4078 | 0.4863 | 0.5124 | 0.044 (2)* | |
| H4B | 0.3434 | 0.4006 | 0.5419 | 0.044 (2)* | |
| N3 | 0.4453 (3) | 0.5149 (5) | 0.61097 (19) | 0.0379 (9) | |
| H3C | 0.4595 | 0.4455 | 0.6448 | 0.044 (2)* | |
| H3D | 0.4073 | 0.5894 | 0.6150 | 0.044 (2)* | |
| H3E | 0.4935 | 0.5660 | 0.6103 | 0.044 (2)* | |
| C5 | 0.3582 (3) | 0.0778 (7) | 0.5890 (2) | 0.0394 (11) | |
| H5A | 0.3532 | −0.0187 | 0.5615 | 0.044 (2)* | |
| H5B | 0.3142 | 0.1572 | 0.5645 | 0.044 (2)* | |
| C6 | 0.3406 (3) | 0.0281 (6) | 0.6498 (3) | 0.0399 (12) | |
| H6A | 0.2837 | −0.0253 | 0.6373 | 0.044 (2)* | |
| H6B | 0.3847 | −0.0512 | 0.6743 | 0.044 (2)* | |
| N4 | 0.3420 (2) | 0.1714 (5) | 0.69203 (18) | 0.0375 (10) | |
| H4C | 0.3941 | 0.2208 | 0.7036 | 0.044 (2)* | |
| H4D | 0.3325 | 0.1373 | 0.7278 | 0.044 (2)* | |
| H4E | 0.3002 | 0.2425 | 0.6702 | 0.044 (2)* | |
| N5 | 0.0735 (2) | 0.5437 (4) | 0.89540 (17) | 0.0257 (8) | |
| C7 | −0.0032 (3) | 0.6180 (6) | 0.9046 (2) | 0.0303 (10) | |
| H7A | 0.0129 | 0.7239 | 0.9263 | 0.044 (2)* | |
| H7B | −0.0232 | 0.5471 | 0.9324 | 0.044 (2)* | |
| C8 | −0.0768 (3) | 0.6422 (6) | 0.8393 (2) | 0.0371 (11) | |
| H8A | −0.1257 | 0.6977 | 0.8462 | 0.044 (2)* | |
| H8B | −0.0566 | 0.7112 | 0.8111 | 0.044 (2)* | |
| N6 | −0.1063 (2) | 0.4800 (5) | 0.80724 (18) | 0.0396 (10) | |
| H6C | −0.0602 | 0.4245 | 0.8049 | 0.044 (2)* | |
| H6D | −0.1447 | 0.4962 | 0.7670 | 0.044 (2)* | |
| H6E | −0.1316 | 0.4222 | 0.8305 | 0.044 (2)* | |
| C9 | 0.1286 (3) | 0.4546 (6) | 0.9547 (2) | 0.0330 (10) | |
| H9A | 0.1348 | 0.5216 | 0.9930 | 0.044 (2)* | |
| H9B | 0.1867 | 0.4386 | 0.9526 | 0.044 (2)* | |
| C10 | 0.0905 (3) | 0.2884 (6) | 0.9620 (2) | 0.0324 (10) | |
| H10A | 0.1238 | 0.2425 | 1.0047 | 0.044 (2)* | |
| H10B | 0.0304 | 0.3030 | 0.9598 | 0.044 (2)* | |
| N7 | 0.0924 (3) | 0.1710 (5) | 0.91024 (19) | 0.0350 (9) | |
| H7C | 0.0633 | 0.2141 | 0.8710 | 0.044 (2)* | |
| H7D | 0.0673 | 0.0763 | 0.9148 | 0.044 (2)* | |
| H7E | 0.1478 | 0.1522 | 0.9138 | 0.044 (2)* | |
| C11 | 0.1253 (3) | 0.6685 (5) | 0.8757 (2) | 0.0309 (10) | |
| H11A | 0.1671 | 0.7186 | 0.9147 | 0.044 (2)* | |
| H11B | 0.0864 | 0.7548 | 0.8508 | 0.044 (2)* | |
| C12 | 0.1743 (3) | 0.5946 (6) | 0.8347 (2) | 0.0319 (10) | |
| H12A | 0.2105 | 0.6789 | 0.8252 | 0.044 (2)* | |
| H12B | 0.2126 | 0.5069 | 0.8592 | 0.044 (2)* | |
| N8 | 0.1116 (2) | 0.5266 (5) | 0.77194 (18) | 0.0329 (9) | |
| H8C | 0.0850 | 0.4378 | 0.7804 | 0.044 (2)* | |
| H8D | 0.1408 | 0.4986 | 0.7458 | 0.044 (2)* | |
| H8E | 0.0716 | 0.6030 | 0.7523 | 0.044 (2)* | |
| F13 | 0.48067 (17) | 0.3777 (3) | 0.73054 (12) | 0.0381 (6) | |
| F14 | 0.00692 (17) | 0.2652 (3) | 0.78508 (13) | 0.0362 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ti1 | 0.0232 (4) | 0.0229 (4) | 0.0258 (4) | 0.0008 (3) | 0.0081 (3) | −0.0022 (3) |
| Ti2 | 0.0313 (4) | 0.0306 (4) | 0.0335 (4) | 0.0001 (4) | 0.0109 (3) | 0.0052 (4) |
| F1 | 0.116 (3) | 0.052 (2) | 0.0285 (15) | 0.000 (2) | 0.0141 (17) | −0.0044 (16) |
| F2 | 0.0296 (15) | 0.0481 (19) | 0.096 (3) | −0.0058 (14) | 0.0206 (16) | 0.0027 (18) |
| F3 | 0.0365 (16) | 0.0480 (18) | 0.088 (2) | −0.0072 (14) | 0.0353 (17) | −0.0135 (17) |
| F4 | 0.0507 (18) | 0.0262 (15) | 0.064 (2) | 0.0073 (13) | 0.0211 (15) | −0.0005 (14) |
| F5 | 0.0505 (17) | 0.0293 (16) | 0.066 (2) | 0.0112 (13) | 0.0185 (15) | 0.0061 (14) |
| F6 | 0.0519 (18) | 0.086 (2) | 0.0284 (15) | −0.0037 (18) | 0.0125 (13) | −0.0022 (16) |
| F7 | 0.0519 (18) | 0.0520 (19) | 0.0443 (17) | −0.0129 (15) | 0.0079 (14) | 0.0156 (15) |
| F8 | 0.088 (2) | 0.055 (2) | 0.0351 (16) | −0.0295 (19) | 0.0079 (16) | 0.0026 (16) |
| F9 | 0.0399 (19) | 0.046 (2) | 0.163 (4) | 0.0094 (16) | 0.041 (2) | 0.025 (2) |
| F10 | 0.0447 (17) | 0.0482 (19) | 0.0568 (18) | 0.0151 (15) | 0.0196 (15) | 0.0040 (16) |
| F11 | 0.071 (2) | 0.0408 (17) | 0.0527 (19) | −0.0096 (16) | 0.0270 (17) | −0.0140 (15) |
| F12 | 0.094 (2) | 0.0410 (17) | 0.0477 (18) | −0.0066 (17) | 0.0445 (18) | 0.0021 (14) |
| N1 | 0.0255 (18) | 0.0327 (19) | 0.0250 (18) | 0.0021 (15) | 0.0096 (15) | −0.0017 (16) |
| C1 | 0.041 (3) | 0.031 (2) | 0.037 (3) | 0.009 (2) | 0.015 (2) | −0.002 (2) |
| C2 | 0.028 (2) | 0.033 (2) | 0.043 (3) | 0.010 (2) | 0.015 (2) | 0.007 (2) |
| N2 | 0.0265 (19) | 0.032 (2) | 0.035 (2) | −0.0022 (16) | 0.0028 (16) | 0.0040 (17) |
| C3 | 0.044 (3) | 0.052 (3) | 0.023 (2) | 0.007 (2) | 0.014 (2) | 0.003 (2) |
| C4 | 0.042 (3) | 0.050 (3) | 0.029 (2) | 0.008 (3) | 0.008 (2) | 0.013 (2) |
| N3 | 0.034 (2) | 0.034 (2) | 0.044 (2) | 0.0034 (18) | 0.0114 (19) | 0.0111 (19) |
| C5 | 0.031 (2) | 0.044 (3) | 0.037 (3) | −0.005 (2) | 0.004 (2) | −0.014 (2) |
| C6 | 0.033 (2) | 0.031 (2) | 0.055 (3) | −0.008 (2) | 0.015 (2) | 0.004 (2) |
| N4 | 0.029 (2) | 0.052 (3) | 0.033 (2) | −0.0063 (19) | 0.0137 (17) | 0.0057 (19) |
| N5 | 0.0284 (18) | 0.0223 (18) | 0.0283 (18) | −0.0008 (14) | 0.0121 (15) | 0.0013 (14) |
| C7 | 0.032 (2) | 0.030 (2) | 0.035 (2) | 0.0019 (19) | 0.0192 (19) | −0.0014 (19) |
| C8 | 0.036 (3) | 0.037 (3) | 0.042 (3) | 0.014 (2) | 0.018 (2) | 0.013 (2) |
| N6 | 0.032 (2) | 0.053 (3) | 0.031 (2) | 0.0091 (19) | 0.0073 (17) | 0.003 (2) |
| C9 | 0.030 (2) | 0.036 (3) | 0.025 (2) | 0.0015 (19) | −0.0001 (18) | −0.0033 (19) |
| C10 | 0.042 (3) | 0.030 (2) | 0.024 (2) | 0.006 (2) | 0.010 (2) | 0.0087 (19) |
| N7 | 0.045 (2) | 0.026 (2) | 0.038 (2) | 0.0029 (18) | 0.0197 (19) | 0.0046 (17) |
| C11 | 0.036 (2) | 0.023 (2) | 0.038 (3) | −0.0053 (19) | 0.019 (2) | −0.0027 (19) |
| C12 | 0.025 (2) | 0.031 (2) | 0.044 (3) | −0.003 (2) | 0.0176 (19) | 0.000 (2) |
| N8 | 0.039 (2) | 0.030 (2) | 0.038 (2) | 0.0009 (17) | 0.0249 (18) | 0.0004 (17) |
| F13 | 0.0377 (15) | 0.0401 (16) | 0.0357 (15) | −0.0005 (12) | 0.0119 (12) | −0.0088 (12) |
| F14 | 0.0398 (15) | 0.0338 (15) | 0.0352 (14) | −0.0026 (12) | 0.0134 (12) | −0.0064 (12) |
Geometric parameters (Å, °)
| Ti1—F1 | 1.796 (3) | C6—N4 | 1.475 (6) |
| Ti1—F2 | 1.826 (3) | C6—H6A | 0.9700 |
| Ti1—F4 | 1.856 (3) | C6—H6B | 0.9700 |
| Ti1—F5 | 1.865 (3) | N4—H4C | 0.8900 |
| Ti1—F3 | 1.868 (3) | N4—H4D | 0.8900 |
| Ti1—F6 | 1.882 (3) | N4—H4E | 0.8900 |
| Ti2—F8 | 1.803 (3) | N5—C7 | 1.459 (5) |
| Ti2—F7 | 1.821 (3) | N5—C11 | 1.472 (5) |
| Ti2—F9 | 1.825 (3) | N5—C9 | 1.475 (5) |
| Ti2—F10 | 1.827 (3) | C7—C8 | 1.516 (6) |
| Ti2—F11 | 1.832 (3) | C7—H7A | 0.9700 |
| Ti2—F12 | 1.856 (3) | C7—H7B | 0.9700 |
| N1—C1 | 1.463 (6) | C8—N6 | 1.484 (6) |
| N1—C5 | 1.470 (6) | C8—H8A | 0.9700 |
| N1—C3 | 1.479 (6) | C8—H8B | 0.9700 |
| C1—C2 | 1.507 (6) | N6—H6C | 0.8900 |
| C1—H1A | 0.9700 | N6—H6D | 0.8900 |
| C1—H1B | 0.9700 | N6—H6E | 0.8900 |
| C2—N2 | 1.487 (6) | C9—C10 | 1.512 (6) |
| C2—H2A | 0.9700 | C9—H9A | 0.9700 |
| C2—H2B | 0.9700 | C9—H9B | 0.9700 |
| N2—H2C | 0.8900 | C10—N7 | 1.483 (6) |
| N2—H2D | 0.8900 | C10—H10A | 0.9700 |
| N2—H2E | 0.8900 | C10—H10B | 0.9700 |
| C3—C4 | 1.512 (7) | N7—H7C | 0.8900 |
| C3—H3A | 0.9700 | N7—H7D | 0.8900 |
| C3—H3B | 0.9700 | N7—H7E | 0.8900 |
| C4—N3 | 1.489 (6) | C11—C12 | 1.513 (6) |
| C4—H4A | 0.9700 | C11—H11A | 0.9700 |
| C4—H4B | 0.9700 | C11—H11B | 0.9700 |
| N3—H3C | 0.8900 | C12—N8 | 1.494 (6) |
| N3—H3D | 0.8900 | C12—H12A | 0.9700 |
| N3—H3E | 0.8900 | C12—H12B | 0.9700 |
| C5—C6 | 1.504 (7) | N8—H8C | 0.8900 |
| C5—H5A | 0.9700 | N8—H8D | 0.8900 |
| C5—H5B | 0.9700 | N8—H8E | 0.8900 |
| F1—Ti1—F2 | 94.09 (17) | N1—C5—H5B | 109.2 |
| F1—Ti1—F4 | 90.38 (15) | C6—C5—H5B | 109.2 |
| F2—Ti1—F4 | 91.12 (14) | H5A—C5—H5B | 107.9 |
| F1—Ti1—F5 | 90.21 (15) | N4—C6—C5 | 111.9 (4) |
| F2—Ti1—F5 | 90.17 (14) | N4—C6—H6A | 109.2 |
| F4—Ti1—F5 | 178.55 (14) | C5—C6—H6A | 109.2 |
| F1—Ti1—F3 | 92.71 (17) | N4—C6—H6B | 109.2 |
| F2—Ti1—F3 | 173.16 (16) | C5—C6—H6B | 109.2 |
| F4—Ti1—F3 | 89.59 (14) | H6A—C6—H6B | 107.9 |
| F5—Ti1—F3 | 89.06 (14) | C6—N4—H4C | 109.5 |
| F1—Ti1—F6 | 178.38 (17) | C6—N4—H4D | 109.5 |
| F2—Ti1—F6 | 87.50 (15) | H4C—N4—H4D | 109.5 |
| F4—Ti1—F6 | 89.26 (15) | C6—N4—H4E | 109.5 |
| F5—Ti1—F6 | 90.11 (15) | H4C—N4—H4E | 109.5 |
| F3—Ti1—F6 | 85.71 (15) | H4D—N4—H4E | 109.5 |
| F8—Ti2—F7 | 93.49 (14) | C7—N5—C11 | 111.2 (3) |
| F8—Ti2—F9 | 90.23 (18) | C7—N5—C9 | 111.6 (3) |
| F7—Ti2—F9 | 91.21 (16) | C11—N5—C9 | 110.8 (3) |
| F8—Ti2—F10 | 90.95 (16) | N5—C7—C8 | 110.9 (4) |
| F7—Ti2—F10 | 89.10 (15) | N5—C7—H7A | 109.5 |
| F9—Ti2—F10 | 178.75 (19) | C8—C7—H7A | 109.5 |
| F8—Ti2—F11 | 175.05 (15) | N5—C7—H7B | 109.5 |
| F7—Ti2—F11 | 91.46 (15) | C8—C7—H7B | 109.5 |
| F9—Ti2—F11 | 89.50 (18) | H7A—C7—H7B | 108.0 |
| F10—Ti2—F11 | 89.29 (15) | N6—C8—C7 | 110.2 (4) |
| F8—Ti2—F12 | 88.59 (14) | N6—C8—H8A | 109.6 |
| F7—Ti2—F12 | 177.05 (15) | C7—C8—H8A | 109.6 |
| F9—Ti2—F12 | 90.86 (17) | N6—C8—H8B | 109.6 |
| F10—Ti2—F12 | 88.78 (15) | C7—C8—H8B | 109.6 |
| F11—Ti2—F12 | 86.47 (14) | H8A—C8—H8B | 108.1 |
| C1—N1—C5 | 111.0 (4) | C8—N6—H6C | 109.5 |
| C1—N1—C3 | 110.0 (4) | C8—N6—H6D | 109.5 |
| C5—N1—C3 | 111.9 (4) | H6C—N6—H6D | 109.5 |
| N1—C1—C2 | 112.9 (4) | C8—N6—H6E | 109.5 |
| N1—C1—H1A | 109.0 | H6C—N6—H6E | 109.5 |
| C2—C1—H1A | 109.0 | H6D—N6—H6E | 109.5 |
| N1—C1—H1B | 109.0 | N5—C9—C10 | 112.4 (3) |
| C2—C1—H1B | 109.0 | N5—C9—H9A | 109.1 |
| H1A—C1—H1B | 107.8 | C10—C9—H9A | 109.1 |
| N2—C2—C1 | 110.8 (4) | N5—C9—H9B | 109.1 |
| N2—C2—H2A | 109.5 | C10—C9—H9B | 109.1 |
| C1—C2—H2A | 109.5 | H9A—C9—H9B | 107.9 |
| N2—C2—H2B | 109.5 | N7—C10—C9 | 111.7 (4) |
| C1—C2—H2B | 109.5 | N7—C10—H10A | 109.3 |
| H2A—C2—H2B | 108.1 | C9—C10—H10A | 109.3 |
| C2—N2—H2C | 109.5 | N7—C10—H10B | 109.3 |
| C2—N2—H2D | 109.5 | C9—C10—H10B | 109.3 |
| H2C—N2—H2D | 109.5 | H10A—C10—H10B | 107.9 |
| C2—N2—H2E | 109.5 | C10—N7—H7C | 109.5 |
| H2C—N2—H2E | 109.5 | C10—N7—H7D | 109.5 |
| H2D—N2—H2E | 109.5 | H7C—N7—H7D | 109.5 |
| N1—C3—C4 | 111.6 (4) | C10—N7—H7E | 109.5 |
| N1—C3—H3A | 109.3 | H7C—N7—H7E | 109.5 |
| C4—C3—H3A | 109.3 | H7D—N7—H7E | 109.5 |
| N1—C3—H3B | 109.3 | N5—C11—C12 | 112.0 (4) |
| C4—C3—H3B | 109.3 | N5—C11—H11A | 109.2 |
| H3A—C3—H3B | 108.0 | C12—C11—H11A | 109.2 |
| N3—C4—C3 | 111.2 (4) | N5—C11—H11B | 109.2 |
| N3—C4—H4A | 109.4 | C12—C11—H11B | 109.2 |
| C3—C4—H4A | 109.4 | H11A—C11—H11B | 107.9 |
| N3—C4—H4B | 109.4 | N8—C12—C11 | 110.7 (3) |
| C3—C4—H4B | 109.4 | N8—C12—H12A | 109.5 |
| H4A—C4—H4B | 108.0 | C11—C12—H12A | 109.5 |
| C4—N3—H3C | 109.5 | N8—C12—H12B | 109.5 |
| C4—N3—H3D | 109.5 | C11—C12—H12B | 109.5 |
| H3C—N3—H3D | 109.5 | H12A—C12—H12B | 108.1 |
| C4—N3—H3E | 109.5 | C12—N8—H8C | 109.5 |
| H3C—N3—H3E | 109.5 | C12—N8—H8D | 109.5 |
| H3D—N3—H3E | 109.5 | H8C—N8—H8D | 109.5 |
| N1—C5—C6 | 112.0 (4) | C12—N8—H8E | 109.5 |
| N1—C5—H5A | 109.2 | H8C—N8—H8E | 109.5 |
| C6—C5—H5A | 109.2 | H8D—N8—H8E | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2C···F13 | 0.89 | 1.90 | 2.773 (5) | 165 |
| N2—H2D···F6i | 0.89 | 2.04 | 2.865 (5) | 154 |
| N2—H2E···F13i | 0.89 | 1.84 | 2.725 (5) | 172 |
| N3—H3C···F13 | 0.89 | 1.86 | 2.700 (5) | 157 |
| N3—H3C···N1 | 0.89 | 2.52 | 2.948 (6) | 110 |
| N3—H3D···F3 | 0.89 | 1.90 | 2.726 (5) | 154 |
| N3—H3E···F9 | 0.89 | 1.84 | 2.717 (5) | 167 |
| N4—H4C···F13 | 0.89 | 1.83 | 2.692 (5) | 162 |
| N4—H4D···F12i | 0.89 | 2.01 | 2.835 (5) | 153 |
| N4—H4E···F5 | 0.89 | 1.84 | 2.712 (5) | 168 |
| N6—H6C···F14 | 0.89 | 1.84 | 2.696 (5) | 162 |
| N6—H6D···F10ii | 0.89 | 2.00 | 2.823 (5) | 154 |
| N6—H6E···F4iii | 0.89 | 1.90 | 2.749 (5) | 160 |
| N7—H7C···F14 | 0.89 | 1.82 | 2.699 (5) | 169 |
| N7—H7D···F2iii | 0.89 | 2.24 | 2.876 (5) | 129 |
| N7—H7E···F7i | 0.89 | 2.08 | 2.916 (5) | 157 |
| N7—H7E···F10i | 0.89 | 2.41 | 2.972 (5) | 121 |
| N8—H8C···F14 | 0.89 | 1.91 | 2.791 (5) | 168 |
| N8—H8D···F6 | 0.89 | 2.14 | 2.879 (5) | 140 |
| N8—H8E···F14iv | 0.89 | 1.81 | 2.702 (5) | 177 |
Symmetry codes: (i) −x+1, y−1/2, −z+3/2; (ii) x−1, y, z; (iii) −x, y−1/2, −z+3/2; (iv) −x, y+1/2, −z+3/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NC2095).
References
- Adil, K., Ben Ali, A., Leblanc, M. & Maisonneuve, V. (2006). Solid State Sci.8, 698–703.
- Allen, F. H., Johnson, O., Shields, G. P., Smith, B. R. & Towler, M. (2004). J. Appl. Cryst.37, 335–338.
- Brandenburg, K. (2001). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Calov, U., Schneider, M. & Leibnitz, P. (1992). Z. Anorg. Allg. Chem.593, 90–98.
- Dadachov, M. S., Tang, L. Q. & Zou, X. D. (2000). Z. Kristallogr. New Cryst. Struct.215, 605–606.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stoe & Cie (1998). STADI4 and X-RED Stoe & Cie, Darmstadt, Germany.
- Tang, L.-Q., Dadachov, M. S. & Zou, X.-D. (2001). Z. Kristallogr. New Cryst. Struct.216, 387–388.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808009781/nc2095sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808009781/nc2095Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report