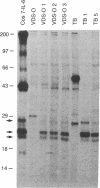
Abstract
Expression of the human IL-6 gene in EBV-immortalized normal human B lymphocytes following retroviral-mediated transduction rendered these cells highly tumorigenic in athymic mice. The tumors were lymphomas composed of the originally inoculated human lymphoblastoid cells. Co-injection of IL-6 expressing EBV-immortalized cells with IL-6 nonexpressing control cells resulted in increased tumorigenicity of the IL-6 nonexpressing cells. The lymphoblastoid cells expressing IL-6 were indistinguishable from parental cell lines in morphology and in a variety of cell surface characteristics, and did not exhibit growth advantage over parental cell lines in vitro, such that increased tumorigenicity is unlikely to depend upon a direct oncogenic effect of IL-6 on the B cells. Rather, at high concentrations, IL-6 markedly inhibits human lymphoblastoid cell killing by IL-2-activated murine splenocytes in vitro, suggesting that IL-6-related tumorigenicity might depend upon IL-6 inhibiting cytotoxicity at the tumor site. Thus, production of IL-6 by tumor cells that results in natural killer cell dysfunctions illustrates a novel mechanism of tumor cell escape from immune surveillance.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Bauer J., Bauer T. M., Kalb T., Taga T., Lengyel G., Hirano T., Kishimoto T., Acs G., Mayer L., Gerok W. Regulation of interleukin 6 receptor expression in human monocytes and monocyte-derived macrophages. Comparison with the expression in human hepatocytes. J Exp Med. 1989 Nov 1;170(5):1537–1549. doi: 10.1084/jem.170.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi A., Rossi V., Bassan R., Barbui T., Bettoni S., Sironi M., Mantovani A., Rambaldi A. Constitutive expression of the interleukin-6 gene in chronic lymphocytic leukemia. Blood. 1989 Apr;73(5):1279–1284. [PubMed] [Google Scholar]
- Brakenhoff J. P., Hart M., De Groot E. R., Di Padova F., Aarden L. A. Structure-function analysis of human IL-6. Epitope mapping of neutralizing monoclonal antibodies with amino- and carboxyl-terminal deletion mutants. J Immunol. 1990 Jul 15;145(2):561–568. [PubMed] [Google Scholar]
- Breen E. C., Rezai A. R., Nakajima K., Beall G. N., Mitsuyasu R. T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- Campbell D. J. Circulating and tissue angiotensin systems. J Clin Invest. 1987 Jan;79(1):1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlin-Henriksson B., Manneborg-Sandlund A., Klein G. Expression of B-cell-specific markers in different Burkitt lymphoma subgroups. Int J Cancer. 1987 Feb 15;39(2):211–218. doi: 10.1002/ijc.2910390215. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Rabinowe S. N., Segil J. M., Horowitz J., Rosen K., Whitman J. F., Nadler L. M. Interleukin 6 gene expression in normal and neoplastic B cells. J Clin Invest. 1989 May;83(5):1512–1518. doi: 10.1172/JCI114046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert D. M., Cancro M. P., Scherle P. A., Nordan R. P., Van Snick J., Gerhard W., Rudikoff S. T cell derived IL-6 is differentially required for antigen-specific antibody secretion by primary and secondary B cells. J Immunol. 1989 Dec 15;143(12):4019–4024. [PubMed] [Google Scholar]
- Hirano T., Taga T., Yasukawa K., Nakajima K., Nakano N., Takatsuki F., Shimizu M., Murashima A., Tsunasawa S., Sakiyama F. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A. 1987 Jan;84(1):228–231. doi: 10.1073/pnas.84.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Jaffe R., Miller G., Breinig M. K., Dummer J. S., Makowka L., Atchison R. W., Karrer F., Nalesnik M. A., Starzl T. E. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988 Apr;45(4):719–727. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacubovich R., Cabrillat H., Gerlier D., Bailly M., Doré J. F. Tumourigenic phenotypes of human melanoma cell lines in nude mice determined by an active antitumour mechanism. Br J Cancer. 1985 Mar;51(3):335–345. doi: 10.1038/bjc.1985.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Content J., Houssiau F., Aarden L., Piechaczyk M., Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989 Feb;73(2):517–526. [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Klein G. Viral latency and transformation: the strategy of Epstein-Barr virus. Cell. 1989 Jul 14;58(1):5–8. doi: 10.1016/0092-8674(89)90394-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luger T. A., Krutmann J., Kirnbauer R., Urbanski A., Schwarz T., Klappacher G., Köck A., Micksche M., Malejczyk J., Schauer E. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J Immunol. 1989 Aug 15;143(4):1206–1209. [PubMed] [Google Scholar]
- Masucci M. G., Bejarano M. T., Masucci G., Klein E. Large granular lymphocytes inhibit the in vitro growth of autologous Epstein-Barr virus-infected B cells. Cell Immunol. 1983 Mar;76(2):311–321. doi: 10.1016/0008-8749(83)90374-x. [DOI] [PubMed] [Google Scholar]
- May L. T., Helfgott D. C., Sehgal P. B. Anti-beta-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the beta 2 interferon involved. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8957–8961. doi: 10.1073/pnas.83.23.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K. H., Giovanella B. C., Klein G., Nilsson K., Stehlin J. S. Diploid human lymphoblastoid and Burkitt lymphoma cell lines: susceptibility to murine NK cells and heterotransplantation to nude mice. Int J Cancer. 1981 Oct 15;28(4):455–458. doi: 10.1002/ijc.2910280410. [DOI] [PubMed] [Google Scholar]
- Miles S. A., Rezai A. R., Salazar-González J. F., Vander Meyden M., Stevens R. H., Logan D. M., Mitsuyasu R. T., Taga T., Hirano T., Kishimoto T. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé J. J., McIntosh J. K., Jablons D. M., Rosenberg S. A. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990 Mar 1;171(3):629–636. doi: 10.1084/jem.171.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Martínez-Maza O., Hirano T., Breen E. C., Nishanian P. G., Salazar-Gonzalez J. F., Fahey J. L., Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989 Jan 15;142(2):531–536. [PubMed] [Google Scholar]
- Nilsson K., Giovanella B. C., Stehlin J. S., Klein G. Tumorigenicity of human hematopoietic cell lines in athymic nude mice. Int J Cancer. 1977 Mar 15;19(3):337–344. doi: 10.1002/ijc.2910190309. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Nordan R. P., Pumphrey J. G., Rudikoff S. Purification and NH2-terminal sequence of a plasmacytoma growth factor derived from the murine macrophage cell line P388D1. J Immunol. 1987 Aug 1;139(3):813–817. [PubMed] [Google Scholar]
- Onozaki K., Matsushima K., Aggarwal B. B., Oppenheim J. J. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985 Dec;135(6):3962–3968. [PubMed] [Google Scholar]
- Patarroyo M., Prieto J., Ernberg I., Gahmberg C. G. Absence, or low expression, of leukocyte adhesion molecules CD11 and CD18 on Burkitt lymphoma cells. Int J Cancer. 1988 Jun 15;41(6):901–907. doi: 10.1002/ijc.2910410623. [DOI] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Arlin Z. A., Wieczorek R., Luciw P., Dina D., Basilico C., Dalla-Favera R. Multiple monoclonal B cell expansions and c-myc oncogene rearrangements in acquired immune deficiency syndrome-related lymphoproliferative disorders. Implications for lymphomagenesis. J Exp Med. 1986 Dec 1;164(6):2049–2060. doi: 10.1084/jem.164.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluda J. M., Yarchoan R., Jaffe E. S., Feuerstein I. M., Solomon D., Steinberg S. M., Wyvill K. M., Raubitschek A., Katz D., Broder S. Development of non-Hodgkin lymphoma in a cohort of patients with severe human immunodeficiency virus (HIV) infection on long-term antiretroviral therapy. Ann Intern Med. 1990 Aug 15;113(4):276–282. doi: 10.7326/0003-4819-113-4-276. [DOI] [PubMed] [Google Scholar]
- Povlsen C. O., Fialkow P. J., Klein E., Klein G., Rygaard J., Wiener F. Growth and antigenic properties of a biopsy-derived Burkitt's lymphoma in thymus-less (nude) mice. Int J Cancer. 1973 Jan 15;11(1):30–39. doi: 10.1002/ijc.2910110105. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T. Opportunistic non-Hodgkin's lymphoma in X-linked recessive immunodeficiency and lymphoproliferative syndromes. Semin Oncol. 1977 Sep;4(3):335–343. [PubMed] [Google Scholar]
- Rooney C. M., Gregory C. D., Rowe M., Finerty S., Edwards C., Rupani H., Rickinson A. B. Endemic Burkitt's lymphoma: phenotypic analysis of tumor biopsy cells and of derived tumor cell lines. J Natl Cancer Inst. 1986 Sep;77(3):681–687. doi: 10.1093/jnci/77.3.681. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rooney C. M., Edwards C. F., Lenoir G. M., Rickinson A. B. Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt's lymphoma. Int J Cancer. 1986 Mar 15;37(3):367–373. doi: 10.1002/ijc.2910370307. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Quinto I., Ruocco M. R., Arcucci A., Mallardo M., Caretto P., Forni G., Venuta S. Expression of an exogenous interleukin 6 gene in human Epstein Barr virus B cells confers growth advantage and in vivo tumorigenicity. J Exp Med. 1990 Jul 1;172(1):61–68. doi: 10.1084/jem.172.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- TERASAKI P. I., MCCLELLAND J. D. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964 Dec 5;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- Taga T., Kawanishi Y., Hardy R. R., Hirano T., Kishimoto T. Receptors for B cell stimulatory factor 2. Quantitation, specificity, distribution, and regulation of their expression. J Exp Med. 1987 Oct 1;166(4):967–981. doi: 10.1084/jem.166.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. E., Goldman N. D., Tosato G. Biochemical and biological analysis of human interleukin 6 expressed in rodent and primate cells. Cytokine. 1990 Sep;2(5):363–374. doi: 10.1016/1043-4666(90)90067-4. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Tosato G., Gerrard T. L., Goldman N. G., Pike S. E. Stimulation of EBV-activated human B cells by monocytes and monocyte products. Role of IFN-beta 2/B cell stimulatory factor 2/IL-6. J Immunol. 1988 Jun 15;140(12):4329–4336. [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Tosato G., Tanner J., Jones K. D., Revel M., Pike S. E. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990 Jun;64(6):3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G. The Epstein-Barr virus and the immune system. Adv Cancer Res. 1987;49:75–125. doi: 10.1016/s0065-230x(08)60795-2. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Shepard H. M., Rothstein J. L., Sugarman B. J., Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Matsuda T., Nishimoto N., Kuritani T., Taeho L., Aozasa K., Nakahata T., Kawai H., Tagoh H., Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989 Sep;74(4):1360–1367. [PubMed] [Google Scholar]
- Ziegler J. L., Beckstead J. A., Volberding P. A., Abrams D. I., Levine A. M., Lukes R. J., Gill P. S., Burkes R. L., Meyer P. R., Metroka C. E. Non-Hodgkin's lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 30;311(9):565–570. doi: 10.1056/NEJM198408303110904. [DOI] [PubMed] [Google Scholar]