Abstract
The title compound, C22H28Br2O2, crystallizes in a staggered arrangement to minimize the interactions of its ortho substituents, with a dihedral angle of 84.2 (3)° between the two aromatic rings. Short C—H⋯O hydrogen-bonding interactions between methoxy groups result in a one-dimensional polymeric chain of molecules lying parallel to the b axis. One tert-butyl group is disordered equally over two positions.
Related literature
For a related structure, see: He & Ng (2006 ▶); Steiner (1996 ▶). For an alternative synthesis, see: Katagiri et al. (2006 ▶).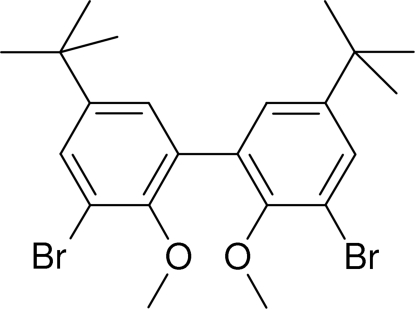
Experimental
Crystal data
C22H28Br2O2
M r = 484.27
Monoclinic,

a = 14.661 (2) Å
b = 13.408 (2) Å
c = 22.489 (3) Å
β = 96.104 (12)°
V = 4395.8 (11) Å3
Z = 8
Mo Kα radiation
μ = 3.70 mm−1
T = 93 (2) K
0.40 × 0.12 × 0.10 mm
Data collection
Bruker APEX2 CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2007 ▶) T min = 0.433, T max = 0.691
14546 measured reflections
3918 independent reflections
2784 reflections with I > 2σ(I)
R int = 0.076
Refinement
R[F 2 > 2σ(F 2)] = 0.067
wR(F 2) = 0.143
S = 1.10
3918 reflections
293 parameters
54 restraints
H-atom parameters constrained
Δρmax = 1.10 e Å−3
Δρmin = −0.93 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Version 1.08; Farrugia, 1997 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808002420/pv2045sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808002420/pv2045Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C31—H31C⋯O10i | 0.98 | 2.61 | 2.842 (15) | 94 |
| C41—H41C⋯O21ii | 0.98 | 2.57 | 2.866 (14) | 98 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
We thank the Foundation of Research Science and Technology for funding. PJS also thanks the Royal Society of New Zealand for the award of a James Cook Research Fellowship.
supplementary crystallographic information
Comment
During attempts to dilithiate 2,6-dibromo-4 - t-butylanisole, the title compound, (I), was serindipitously produced. Compared to the literature methods (Katagiri et al., 2006) this is a much simpler method, wherein the product was acheived in a single step rather than three and with a superior overall yield. The structure adopts a staggered arrangement with a dihedral angle of 84.2 (3)° between the two aromatic rings. A similar angle is found in the literature (80.1°, He & Ng, 2006). Two CH3···O hydrogen bonds involving both methoxy groups (Table 1) connect the molecules to form a one dimensional polymeric chain parallel to the b axis (Figure 2); similar type of interactions have already been reported (Steiner, 1996).
Experimental
2,6-Dibromo-4 - t-butylanisole (1 g) in THF (40 ml) at 193 K was treated with n-butyl lithium (1.6 M, 2.5 ml). The solution was stirred and allowed to warm up to room temp over 2 hr. The resulting solution was evaporated to dryness, treated with water and extracted with dichloromethane. The organic layer was seperated and purified by column chromotography (SiO2, dichloromethane). Yeild = 0.6 g (79%).
Refinement
The methoxy groups are both evenly disordered over two sites. One tert-butyl group is disordered over two sites whilst the other is not. This breaks the potential symmetry between the two halves of the molecule. Both tert-butyl groups exhibited elongation of the thermal elipsoids and have been restrained (ISOR) to be more isotropic. The large redidual electron density (1.11 e/A*3) is located 0.64Å from H70A and is probably related to a small amount of unmodelled tert-butyl group disorder. All H-atoms were positioned geometrically and refined using a riding model with d(C—H) = 0.95 Å, Uiso = 1.2Ueq(C) for aromatic, and 0.98 Å, Uiso = 1.5Ueq(C) for CH3 atoms.
Figures
Fig. 1.
A view of the asymmetric unit of (I), showing displacement ellipsoids at the 50% probability level. All hydrogen atoms have been omited for clarity. The bonds for one of the disordered parts are displayed as hollow bonds.
Fig. 2.
A diagram showing the hydrogen bonding which extends the structure into a 1-D polymer. The closest C—H···O bonds are shown as dashed lines.
Crystal data
| C22H28Br2O2 | F000 = 1968 |
| Mr = 484.27 | Dx = 1.463 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 4881 reflections |
| a = 14.661 (2) Å | θ = 2.8–26.5º |
| b = 13.408 (2) Å | µ = 3.70 mm−1 |
| c = 22.489 (3) Å | T = 93 (2) K |
| β = 96.104 (12)º | Shard, colourless |
| V = 4395.8 (11) Å3 | 0.40 × 0.12 × 0.10 mm |
| Z = 8 |
Data collection
| Bruker APEX2 CCD area-detector diffractometer | 3918 independent reflections |
| Radiation source: sealed tube | 2784 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.077 |
| T = 93(2) K | θmax = 25.1º |
| φ and ω scans | θmin = 2.1º |
| Absorption correction: multi-scan(SADABS; Bruker, 2007) | h = −17→17 |
| Tmin = 0.433, Tmax = 0.691 | k = −14→16 |
| 14546 measured reflections | l = −18→26 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.067 | H-atom parameters constrained |
| wR(F2) = 0.143 | w = 1/[σ2(Fo2) + (0.0562P)2 + 0.5007P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.10 | (Δ/σ)max < 0.001 |
| 3918 reflections | Δρmax = 1.11 e Å−3 |
| 293 parameters | Δρmin = −0.93 e Å−3 |
| 54 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Experimental. Spectroscopic data: 1H NMR (CDCl3): δ 1.33 (18H, s, (CH3)3), 3.53 (6H, s, OCH3), 7.36 (2H, d, ArH), 7.57 (2H, d, ArH). Mass Spec: (ESI-TOF) 485.3 {M+} calc 485.05. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Br10 | 0.15107 (7) | 0.73599 (8) | 0.05847 (4) | 0.0641 (3) | |
| C10 | 0.2414 (5) | 0.7782 (5) | 0.1739 (3) | 0.0318 (16) | |
| C11 | 0.2365 (5) | 0.8029 (6) | 0.1143 (3) | 0.0384 (18) | |
| C12 | 0.2911 (5) | 0.8769 (6) | 0.0943 (3) | 0.0395 (18) | |
| H12 | 0.2846 | 0.8937 | 0.0531 | 0.047* | |
| C13 | 0.3553 (5) | 0.9273 (5) | 0.1330 (3) | 0.0319 (16) | |
| C14 | 0.3623 (5) | 0.8991 (5) | 0.1932 (3) | 0.0305 (16) | |
| H14 | 0.4073 | 0.9302 | 0.2206 | 0.037* | |
| C15 | 0.3053 (4) | 0.8267 (5) | 0.2143 (3) | 0.0278 (15) | |
| C16 | 0.4181 (6) | 1.0090 (5) | 0.1119 (3) | 0.0404 (18) | |
| Br21 | 0.18307 (6) | 0.87188 (6) | 0.42817 (3) | 0.0464 (2) | |
| C20 | 0.3129 (4) | 0.7995 (5) | 0.2790 (3) | 0.0264 (15) | |
| C21 | 0.2538 (4) | 0.8409 (5) | 0.3166 (3) | 0.0264 (15) | |
| C22 | 0.2626 (4) | 0.8137 (5) | 0.3765 (3) | 0.0268 (15) | |
| C23 | 0.3269 (4) | 0.7445 (5) | 0.3990 (3) | 0.0285 (15) | |
| H23 | 0.3308 | 0.7263 | 0.4400 | 0.034* | |
| C24 | 0.3863 (4) | 0.7008 (5) | 0.3618 (3) | 0.0247 (14) | |
| C25 | 0.3790 (4) | 0.7306 (5) | 0.3025 (3) | 0.0265 (14) | |
| H25 | 0.4202 | 0.7035 | 0.2769 | 0.032* | |
| C26 | 0.4557 (5) | 0.6205 (5) | 0.3860 (3) | 0.0317 (16) | |
| O21 | 0.1887 (3) | 0.9095 (3) | 0.2945 (2) | 0.0332 (11) | |
| C30 | 0.0955 (9) | 0.8615 (11) | 0.2798 (7) | 0.042 (4) | 0.50 |
| H30A | 0.1009 | 0.8049 | 0.2528 | 0.062* | 0.50 |
| H30B | 0.0526 | 0.9105 | 0.2603 | 0.062* | 0.50 |
| H30C | 0.0726 | 0.8379 | 0.3167 | 0.062* | 0.50 |
| C31 | 0.2269 (10) | 1.0158 (10) | 0.2987 (6) | 0.037 (3) | 0.50 |
| H31A | 0.2469 | 1.0319 | 0.3405 | 0.055* | 0.50 |
| H31B | 0.1790 | 1.0628 | 0.2830 | 0.055* | 0.50 |
| H31C | 0.2792 | 1.0208 | 0.2751 | 0.055* | 0.50 |
| O10 | 0.1857 (3) | 0.7052 (3) | 0.1937 (2) | 0.0349 (11) | |
| C40 | 0.0985 (9) | 0.7443 (12) | 0.2011 (7) | 0.043 (4) | 0.50 |
| H40A | 0.1045 | 0.7958 | 0.2322 | 0.064* | 0.50 |
| H40B | 0.0587 | 0.6908 | 0.2130 | 0.064* | 0.50 |
| H40C | 0.0716 | 0.7736 | 0.1634 | 0.064* | 0.50 |
| C41 | 0.2209 (10) | 0.5993 (9) | 0.1949 (6) | 0.036 (3) | 0.50 |
| H41A | 0.2306 | 0.5786 | 0.1542 | 0.054* | 0.50 |
| H41B | 0.1760 | 0.5550 | 0.2105 | 0.054* | 0.50 |
| H41C | 0.2791 | 0.5955 | 0.2206 | 0.054* | 0.50 |
| C50 | 0.4475 (12) | 0.5886 (13) | 0.4517 (7) | 0.050 (4) | 0.50 |
| H50A | 0.4877 | 0.5314 | 0.4619 | 0.075* | 0.50 |
| H50B | 0.3838 | 0.5699 | 0.4559 | 0.075* | 0.50 |
| H50C | 0.4657 | 0.6442 | 0.4786 | 0.075* | 0.50 |
| C51 | 0.4383 (12) | 0.5241 (12) | 0.3484 (7) | 0.052 (4) | 0.50 |
| H51A | 0.4576 | 0.5346 | 0.3085 | 0.078* | 0.50 |
| H51B | 0.3728 | 0.5078 | 0.3449 | 0.078* | 0.50 |
| H51C | 0.4735 | 0.4690 | 0.3682 | 0.078* | 0.50 |
| C52 | 0.5535 (11) | 0.6565 (13) | 0.3834 (8) | 0.053 (4) | 0.50 |
| H52A | 0.5635 | 0.7177 | 0.4070 | 0.080* | 0.50 |
| H52B | 0.5636 | 0.6699 | 0.3418 | 0.080* | 0.50 |
| H52C | 0.5965 | 0.6050 | 0.3999 | 0.080* | 0.50 |
| C60 | 0.4038 (11) | 0.5287 (11) | 0.3985 (7) | 0.043 (4) | 0.50 |
| H60A | 0.3881 | 0.4918 | 0.3612 | 0.065* | 0.50 |
| H60B | 0.3474 | 0.5473 | 0.4155 | 0.065* | 0.50 |
| H60C | 0.4416 | 0.4866 | 0.4269 | 0.065* | 0.50 |
| C61 | 0.5257 (11) | 0.6015 (12) | 0.3413 (7) | 0.045 (4) | 0.50 |
| H61A | 0.5465 | 0.6654 | 0.3264 | 0.067* | 0.50 |
| H61B | 0.4971 | 0.5619 | 0.3077 | 0.067* | 0.50 |
| H61C | 0.5784 | 0.5651 | 0.3612 | 0.067* | 0.50 |
| C62 | 0.5094 (10) | 0.6633 (11) | 0.4442 (6) | 0.038 (3) | 0.50 |
| H62A | 0.5527 | 0.6131 | 0.4618 | 0.057* | 0.50 |
| H62B | 0.4661 | 0.6806 | 0.4728 | 0.057* | 0.50 |
| H62C | 0.5431 | 0.7232 | 0.4343 | 0.057* | 0.50 |
| C70 | 0.4117 (9) | 1.0992 (9) | 0.1512 (5) | 0.103 (4) | |
| H70A | 0.4498 | 1.0889 | 0.1892 | 0.155* | |
| H70B | 0.3477 | 1.1092 | 0.1588 | 0.155* | |
| H70C | 0.4333 | 1.1582 | 0.1311 | 0.155* | |
| C71 | 0.3892 (7) | 1.0442 (8) | 0.0495 (4) | 0.079 (3) | |
| H71A | 0.4268 | 1.1015 | 0.0404 | 0.119* | |
| H71B | 0.3245 | 1.0640 | 0.0461 | 0.119* | |
| H71C | 0.3973 | 0.9902 | 0.0212 | 0.119* | |
| C72 | 0.5157 (6) | 0.9717 (7) | 0.1155 (4) | 0.065 (3) | |
| H72A | 0.5559 | 1.0256 | 0.1047 | 0.098* | |
| H72B | 0.5193 | 0.9157 | 0.0879 | 0.098* | |
| H72C | 0.5353 | 0.9495 | 0.1564 | 0.098* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br10 | 0.0766 (7) | 0.0720 (7) | 0.0396 (5) | −0.0096 (5) | −0.0125 (4) | −0.0105 (5) |
| C10 | 0.030 (4) | 0.038 (4) | 0.027 (4) | 0.015 (3) | 0.003 (3) | 0.001 (3) |
| C11 | 0.040 (4) | 0.046 (5) | 0.029 (4) | 0.013 (4) | −0.002 (3) | −0.007 (3) |
| C12 | 0.051 (5) | 0.049 (5) | 0.019 (3) | 0.008 (4) | 0.006 (3) | 0.004 (3) |
| C13 | 0.035 (4) | 0.034 (4) | 0.027 (4) | 0.008 (3) | 0.005 (3) | 0.003 (3) |
| C14 | 0.033 (4) | 0.028 (4) | 0.032 (4) | 0.002 (3) | 0.009 (3) | 0.001 (3) |
| C15 | 0.028 (4) | 0.026 (4) | 0.030 (4) | 0.010 (3) | 0.007 (3) | 0.000 (3) |
| C16 | 0.065 (5) | 0.029 (4) | 0.029 (4) | 0.004 (4) | 0.012 (4) | 0.004 (3) |
| Br21 | 0.0562 (5) | 0.0481 (5) | 0.0387 (4) | 0.0201 (4) | 0.0232 (3) | 0.0043 (4) |
| C20 | 0.026 (4) | 0.028 (4) | 0.025 (3) | −0.003 (3) | 0.002 (3) | 0.000 (3) |
| C21 | 0.020 (3) | 0.021 (3) | 0.039 (4) | 0.001 (3) | 0.007 (3) | 0.003 (3) |
| C22 | 0.026 (4) | 0.026 (4) | 0.029 (4) | 0.002 (3) | 0.004 (3) | −0.001 (3) |
| C23 | 0.035 (4) | 0.028 (4) | 0.023 (3) | −0.005 (3) | 0.007 (3) | −0.003 (3) |
| C24 | 0.028 (4) | 0.022 (3) | 0.024 (3) | −0.002 (3) | 0.005 (3) | 0.001 (3) |
| C25 | 0.028 (4) | 0.028 (4) | 0.023 (3) | −0.001 (3) | 0.001 (3) | 0.000 (3) |
| C26 | 0.035 (4) | 0.033 (4) | 0.028 (4) | 0.008 (3) | 0.006 (3) | 0.008 (3) |
| O21 | 0.032 (3) | 0.027 (3) | 0.041 (3) | 0.004 (2) | 0.008 (2) | 0.007 (2) |
| C30 | 0.035 (8) | 0.034 (9) | 0.054 (9) | 0.008 (7) | −0.009 (7) | 0.008 (7) |
| C31 | 0.057 (10) | 0.033 (8) | 0.022 (7) | 0.006 (7) | 0.009 (7) | −0.003 (6) |
| O10 | 0.033 (3) | 0.034 (3) | 0.038 (3) | 0.002 (2) | 0.006 (2) | 0.000 (2) |
| C40 | 0.027 (8) | 0.053 (10) | 0.046 (9) | −0.008 (7) | −0.004 (6) | 0.000 (8) |
| C41 | 0.039 (8) | 0.022 (8) | 0.047 (9) | −0.013 (6) | 0.004 (7) | 0.005 (6) |
| C50 | 0.059 (8) | 0.052 (8) | 0.039 (7) | 0.015 (7) | 0.008 (6) | 0.018 (6) |
| C51 | 0.062 (8) | 0.040 (7) | 0.050 (7) | 0.015 (7) | −0.006 (7) | −0.001 (6) |
| C52 | 0.045 (7) | 0.056 (8) | 0.059 (8) | 0.007 (7) | 0.008 (6) | 0.015 (7) |
| C60 | 0.047 (7) | 0.031 (7) | 0.052 (7) | −0.004 (6) | 0.004 (6) | 0.008 (6) |
| C61 | 0.042 (7) | 0.053 (8) | 0.039 (7) | 0.015 (6) | 0.007 (6) | 0.000 (6) |
| C62 | 0.046 (7) | 0.038 (7) | 0.029 (6) | 0.006 (6) | 0.001 (6) | 0.014 (6) |
| C70 | 0.126 (6) | 0.085 (5) | 0.104 (5) | −0.007 (4) | 0.035 (4) | 0.003 (4) |
| C71 | 0.075 (5) | 0.090 (5) | 0.072 (5) | −0.009 (4) | 0.010 (4) | 0.027 (4) |
| C72 | 0.059 (4) | 0.058 (4) | 0.078 (4) | −0.011 (4) | 0.005 (4) | 0.019 (4) |
Geometric parameters (Å, °)
| Br10—C11 | 1.902 (7) | C31—H31B | 0.9800 |
| C10—C11 | 1.375 (9) | C31—H31C | 0.9800 |
| C10—O10 | 1.379 (8) | O10—C40 | 1.408 (15) |
| C10—C15 | 1.394 (9) | O10—C41 | 1.511 (14) |
| C11—C12 | 1.379 (10) | C40—H40A | 0.9800 |
| C12—C13 | 1.388 (10) | C40—H40B | 0.9800 |
| C12—H12 | 0.9500 | C40—H40C | 0.9800 |
| C13—C14 | 1.398 (9) | C41—H41A | 0.9800 |
| C13—C16 | 1.539 (10) | C41—H41B | 0.9800 |
| C14—C15 | 1.397 (9) | C41—H41C | 0.9800 |
| C14—H14 | 0.9500 | C50—H50A | 0.9800 |
| C15—C20 | 1.493 (9) | C50—H50B | 0.9800 |
| C16—C71 | 1.499 (11) | C50—H50C | 0.9800 |
| C16—C70 | 1.507 (13) | C51—H51A | 0.9800 |
| C16—C72 | 1.510 (11) | C51—H51B | 0.9800 |
| Br21—C22 | 1.899 (6) | C51—H51C | 0.9800 |
| C20—C21 | 1.390 (9) | C52—H52A | 0.9800 |
| C20—C25 | 1.401 (9) | C52—H52B | 0.9800 |
| C21—O21 | 1.380 (7) | C52—H52C | 0.9800 |
| C21—C22 | 1.388 (9) | C60—H60A | 0.9800 |
| C22—C23 | 1.380 (9) | C60—H60B | 0.9800 |
| C23—C24 | 1.398 (9) | C60—H60C | 0.9800 |
| C23—H23 | 0.9500 | C61—H61A | 0.9800 |
| C24—C25 | 1.385 (8) | C61—H61B | 0.9800 |
| C24—C26 | 1.541 (9) | C61—H61C | 0.9800 |
| C25—H25 | 0.9500 | C62—H62A | 0.9800 |
| C26—C60 | 1.489 (15) | C62—H62B | 0.9800 |
| C26—C52 | 1.519 (17) | C62—H62C | 0.9800 |
| C26—C61 | 1.533 (15) | C70—H70A | 0.9800 |
| C26—C51 | 1.550 (17) | C70—H70B | 0.9800 |
| C26—C50 | 1.555 (15) | C70—H70C | 0.9800 |
| C26—C62 | 1.563 (15) | C71—H71A | 0.9800 |
| O21—C30 | 1.516 (14) | C71—H71B | 0.9800 |
| O21—C31 | 1.530 (15) | C71—H71C | 0.9800 |
| C30—H30A | 0.9800 | C72—H72A | 0.9800 |
| C30—H30B | 0.9800 | C72—H72B | 0.9800 |
| C30—H30C | 0.9800 | C72—H72C | 0.9800 |
| C31—H31A | 0.9800 | ||
| C11—C10—O10 | 121.0 (6) | O21—C30—H30A | 109.5 |
| C11—C10—C15 | 119.0 (7) | O21—C30—H30B | 109.5 |
| O10—C10—C15 | 120.0 (6) | O21—C30—H30C | 109.5 |
| C10—C11—C12 | 121.3 (7) | O21—C31—H31A | 109.5 |
| C10—C11—Br10 | 119.5 (6) | O21—C31—H31B | 109.5 |
| C12—C11—Br10 | 119.2 (5) | H31A—C31—H31B | 109.5 |
| C11—C12—C13 | 121.6 (6) | O21—C31—H31C | 109.5 |
| C11—C12—H12 | 119.2 | H31A—C31—H31C | 109.5 |
| C13—C12—H12 | 119.2 | H31B—C31—H31C | 109.5 |
| C12—C13—C14 | 116.8 (6) | C10—O10—C40 | 110.5 (8) |
| C12—C13—C16 | 122.8 (6) | C10—O10—C41 | 117.3 (7) |
| C14—C13—C16 | 120.5 (6) | C40—O10—C41 | 131.4 (9) |
| C15—C14—C13 | 122.1 (6) | O10—C40—H40A | 109.5 |
| C15—C14—H14 | 118.9 | O10—C40—H40B | 109.5 |
| C13—C14—H14 | 118.9 | H40A—C40—H40B | 109.5 |
| C10—C15—C14 | 119.2 (6) | O10—C40—H40C | 109.5 |
| C10—C15—C20 | 119.8 (6) | H40A—C40—H40C | 109.5 |
| C14—C15—C20 | 121.1 (6) | H40B—C40—H40C | 109.5 |
| C71—C16—C70 | 105.5 (8) | O10—C41—H41A | 109.5 |
| C71—C16—C72 | 109.2 (7) | O10—C41—H41B | 109.5 |
| C70—C16—C72 | 110.6 (8) | O10—C41—H41C | 109.5 |
| C71—C16—C13 | 113.3 (7) | C26—C50—H50A | 109.5 |
| C70—C16—C13 | 108.3 (7) | C26—C50—H50B | 109.5 |
| C72—C16—C13 | 110.0 (6) | C26—C50—H50C | 109.5 |
| C21—C20—C25 | 119.0 (6) | C26—C51—H51A | 109.5 |
| C21—C20—C15 | 120.6 (6) | C26—C51—H51B | 109.5 |
| C25—C20—C15 | 120.4 (6) | C26—C51—H51C | 109.5 |
| O21—C21—C22 | 121.0 (5) | C26—C52—H52A | 109.5 |
| O21—C21—C20 | 120.0 (6) | C26—C52—H52B | 109.5 |
| C22—C21—C20 | 119.0 (6) | C26—C52—H52C | 109.5 |
| C23—C22—C21 | 121.5 (6) | C26—C60—H60A | 109.5 |
| C23—C22—Br21 | 119.5 (5) | C26—C60—H60B | 109.5 |
| C21—C22—Br21 | 119.0 (5) | H60A—C60—H60B | 109.5 |
| C22—C23—C24 | 120.4 (6) | C26—C60—H60C | 109.5 |
| C22—C23—H23 | 119.8 | H60A—C60—H60C | 109.5 |
| C24—C23—H23 | 119.8 | H60B—C60—H60C | 109.5 |
| C25—C24—C23 | 117.8 (6) | C26—C61—H61A | 109.5 |
| C25—C24—C26 | 121.5 (5) | C26—C61—H61B | 109.5 |
| C23—C24—C26 | 120.7 (5) | H61A—C61—H61B | 109.5 |
| C24—C25—C20 | 122.2 (6) | C26—C61—H61C | 109.5 |
| C24—C25—H25 | 118.9 | H61A—C61—H61C | 109.5 |
| C20—C25—H25 | 118.9 | H61B—C61—H61C | 109.5 |
| C60—C26—C52 | 140.6 (10) | C26—C62—H62A | 109.5 |
| C60—C26—C61 | 112.2 (10) | C26—C62—H62B | 109.5 |
| C52—C26—C61 | 47.7 (9) | H62A—C62—H62B | 109.5 |
| C60—C26—C24 | 108.2 (8) | C26—C62—H62C | 109.5 |
| C52—C26—C24 | 110.8 (8) | H62A—C62—H62C | 109.5 |
| C61—C26—C24 | 110.4 (7) | H62B—C62—H62C | 109.5 |
| C60—C26—C51 | 49.9 (9) | C16—C70—H70A | 109.5 |
| C52—C26—C51 | 110.2 (11) | C16—C70—H70B | 109.5 |
| C61—C26—C51 | 65.6 (10) | H70A—C70—H70B | 109.5 |
| C24—C26—C51 | 109.0 (8) | C16—C70—H70C | 109.5 |
| C60—C26—C50 | 60.0 (9) | H70A—C70—H70C | 109.5 |
| C52—C26—C50 | 107.0 (10) | H70B—C70—H70C | 109.5 |
| C61—C26—C50 | 134.6 (9) | C16—C71—H71A | 109.5 |
| C24—C26—C50 | 114.4 (7) | C16—C71—H71B | 109.5 |
| C51—C26—C50 | 105.3 (10) | H71A—C71—H71B | 109.5 |
| C60—C26—C62 | 111.6 (9) | C16—C71—H71C | 109.5 |
| C52—C26—C62 | 61.4 (9) | H71A—C71—H71C | 109.5 |
| C61—C26—C62 | 107.3 (9) | H71B—C71—H71C | 109.5 |
| C24—C26—C62 | 107.1 (7) | C16—C72—H72A | 109.5 |
| C51—C26—C62 | 143.3 (9) | C16—C72—H72B | 109.5 |
| C50—C26—C62 | 52.4 (8) | H72A—C72—H72B | 109.5 |
| C21—O21—C30 | 111.6 (7) | C16—C72—H72C | 109.5 |
| C21—O21—C31 | 111.4 (7) | H72A—C72—H72C | 109.5 |
| C30—O21—C31 | 136.5 (8) | H72B—C72—H72C | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C31—H31C···O10i | 0.98 | 2.61 | 2.842 (15) | 94 |
| C41—H41C···O21ii | 0.98 | 2.57 | 2.866 (14) | 98 |
Symmetry codes: (i) −x+1/2, y+1/2, −z+1/2; (ii) −x+1/2, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PV2045).
References
- Bruker (2007). APEX2 (Version 2.1-4), SAINT (Version 7.34A), SADABS (Version 2004/1). Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- He, L. & Ng, S. W. (2006). Acta Cryst. E62, o5517–o5519.
- Katagiri, H., Miyagawa, T., Furusho, Y. & Yashima, E. (2006). Angew. Chem. Int. Ed.45, 1741–1744. [DOI] [PubMed]
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Steiner, T. (1996). Cryst. Rev.6, 1–57.
- Westrip, S. P. (2008). publCIF. In preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808002420/pv2045sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808002420/pv2045Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




