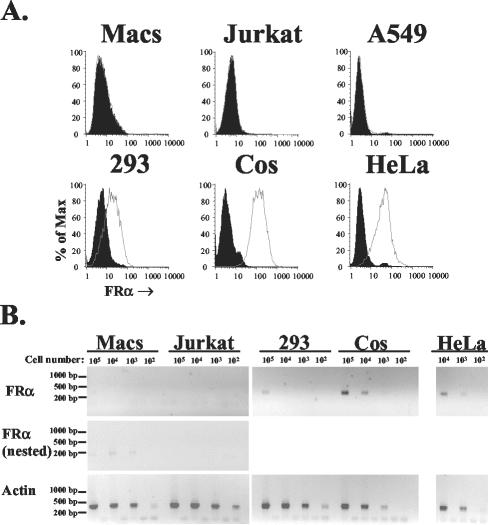
FIG. 1.
FRα expression on common cell lines and primary macrophages. (A) Surface levels of FRα on five cell lines and day 6 primary macrophages were determined by standard flow cytometry (33) with a murine monoclonal antibody directed against human FRα (LK26; Signet) (open histograms) and an isotype control (immunoglobulin G2a; Sigma, St. Louis, Mo.) (filled histograms). Macrophages were isolated from human leukocytes by plastic adherence, as previously described (32). (B) Levels of cellular mRNA for FRα and human β-actin were evaluated by RT-PCR, using an Express direct mRNA capture and RT system (Pierce, Rockford, Ill.). mRNA was extracted and cDNA synthesis was performed in the same well containing serial 10-fold dilutions of each cell type. PCR was performed with 16.5 μl of cDNA by using the conditions 95°C for 30 s followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 40 s for FRα (forward primer, TGGGTGGCTGTAGTAGGGGAG; reverse primer, CAGGGGCACGTTCAGTACC) and β-actin (forward primer, CTGGCACCACACCTTCTACAATG; reverse primer, AATGTCACGCACGATTTCCCGC), resulting in 359- and 381-bp products, respectively. Primers were designed so that products spanned intron and exon boundaries, such that the predicted products derived from genomic DNAs were 3,440 and 822 bp for FRα and β-actin, respectively. For cell types negative for FRα in the first round of PCR (macrophages [Macs] and the T-cell line, Jurkat), 5 μl was transferred to new tubes and nested PCR was performed with the same conditions as those described above and the following set of nested primers: forward primer GCCAAGCACCACAAGGAAAAG and reverse primer CCTGGATGAAATGCCGTTTG. This resulted in a 189-bp product with a 3,118-bp predicted genomic DNA product. Both first-round and nested primers recognized all seven isoforms of FRα (8). Gaps between each sample represent controls for which the whole mRNA extraction and RT-PCR procedure was carried out on empty wells (a total of seven controls for the nested PCR). Control experiments with the same reaction mixtures performed on mRNA from each cell line in the absence of reverse transcriptase were all negative (data not shown).