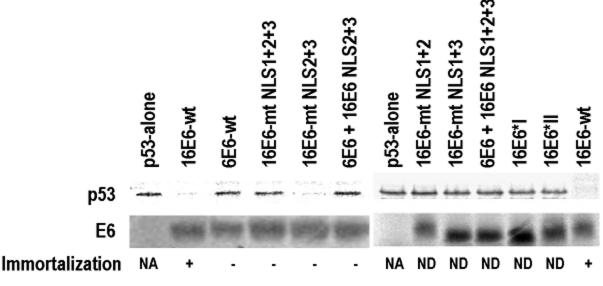
FIG. 9.
16E6 NLS sequences are important in the degradation of p53 in vitro and in the immortalization of human MECs. E6 and p53 proteins were prepared with a rabbit reticulocyte lysate transcription-translation system in the presence of [35S]methionine. The in vitro p53 degradation assay was described by Liu et al. (41). To minimize the background, p53 polyclonal antibody was used to immunoprecipitate p53 from the degradation reaction mixture. Briefly, 10 μl of p53 degradation solution, 25 μl of protein G-Sepharose beads, and 100 μl of p53 antibody were mixed with 400 μl of binding buffer (100 mM NaCl, 100 mM Tris-HCl [pH 8.0], 0.5% EDTA, 0.5% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, 0.5% protease inhibitor cocktail [Roche]). The mixture was rotated at 4°C overnight and washed three times with binding buffer. The immunoprecipitated proteins were then resolved on an SDS-12% polyacrylamide gel and visualized by autoradiography. Individual proteins used for p53 degradation are indicated above the lanes. The 16E6 NLS sequences with mutations are shown in Fig. 7, and their substitutions for the corresponding regions in 6E6 are shown in Fig. 8. The 16E6 mutant (16E6-mt) NLS lanes indicate combined mutations of the individual NLS sequences. The chimeric 6E6-16E6 (6E6 + 16E6) NLS lane indicates the corresponding regions of 6E6 being replaced by 16E6 NLS sequences. Shown below the p53 gel are the corresponding E6 proteins with (+) or without (−) immortalization competency, as examined in human MECs. wt, wild type; NA, not applicable; ND, not done.