Abstract
The title compound, C15H16N2O5, belongs to the class of monastrol-type anti-cancer agents and was selected for crystal structure determination in order to determine the conformational details needed for subsequent structure–activity relationship studies. The central tetrahydropyrimidine ring has a flat-envelope conformation. The 4-phenyl group occupies a pseudo-axial position and is inclined at an angle of ca 90° to the mean plane of the heterocyclic ring. Of the two methyl ester groups, one (in the 5-position) is in a coplanar and the other (in the 6-position) in a perpendicular orientation with respect to the heterocyclic plane. The coplanar 5-ester group has its carbonyl bond oriented cis with respect to the pyrimidine C=C double bond. By comparison of the structural results for the present compound with those determined previously for its diethyl analogue, we have identified the molecular factors which control the dual course of the Biginelli reaction with salicylaldehyde. The crystal structure is dominated by two hydrogen bonds which link the molecules into chains of dimers.
Related literature
For related literature, see: Haggarty et al. (2000 ▶); Hirshfeld (1976 ▶); Kettmann et al. (2008 ▶); Klein et al. (2007 ▶); Mayer et al. (1999 ▶); Světlík et al. (2008 ▶).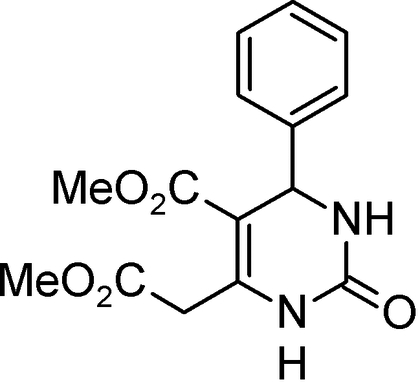
Experimental
Crystal data
C15H16N2O5
M r = 304.35
Monoclinic,

a = 23.498 (5) Å
b = 12.072 (2) Å
c = 10.933 (5) Å
β = 99.15 (2)°
V = 3061.9 (16) Å3
Z = 8
Mo Kα radiation
μ = 0.10 mm−1
T = 296 (2) K
0.30 × 0.25 × 0.20 mm
Data collection
Siemens P4 diffractometer
Absorption correction: none
5187 measured reflections
4466 independent reflections
2308 reflections with I > 2σ(I)
R int = 0.040
3 standard reflections every 97 reflections intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.046
wR(F 2) = 0.118
S = 0.90
4466 reflections
201 parameters
54 restraints
H-atom parameters constrained
Δρmax = 0.13 e Å−3
Δρmin = −0.27 e Å−3
Data collection: XSCANS (Siemens, 1991 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2003 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808026135/bv2102sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808026135/bv2102Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1i | 0.86 | 2.06 | 2.8326 (17) | 149 |
| N3—H3⋯O4ii | 0.86 | 2.32 | 3.0730 (17) | 146 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
This work was supported by the Grant Agency of the Slovak Republic, project Nos. 1/4298/07 and 1/4299/07.
supplementary crystallographic information
Comment
Recently, the discovery of monastrol [ethyl 6-methyl-4-(3-hydroxyphenyl)- 2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate] (Mayer et al., 1999), a lead structure for development of new anticancer agents (Klein et al., 2007), has stimulated considerable interest in preparing its congeners (Haggarty et al., 2000). To follow this research, our strategy was to synthesize conformationally restricted dihydropyrimidine heterocycles by employing a Biginelli-like condensation of salicylaldehyde with dialkyl acetone-1,3-dicarboxylates (Světlík et al., 2008). Unexpectedly, formation of two types of products was detected depending on the ester alkyl group of the starting dialkyl 3-oxopentanedioate: for the methyl ester the reaction proceeded in a 'normal' way to produce the rigid tricyclic structure (3), while the ethyl analogue gave the structure (2), i.e. the final cyclization did not occur. To provide structural basis for this product dichotomy, we selected compounds (1) and (2) for a single-crystal X-ray analysis. As the structure of (2) was described previously (Kettmann et al., 2008), we report herein the structure of the title compound (1); it is the de-hydroxy derivative (to prevent cyclization of the molecule) of its normal tricyclic compound.
As mentioned above, the analysis of the structural results (Fig.1, Table 1) is focused on the conformational characteristics of the present structure (1) in comparison with those obtained recently for (2). The comparison has shown that, apart from the conformation of the 5-ester substituent (cis and trans in (1) and (2), respectively), the only significant difference between (1) and (2) concerns rotation around the ROOC-CH2 single bond as measured by the torsion angle C6—C15—C16—O4 which is ca 0° in (1) and ca 180° in (2). As this is the only difference which affect the vicinity of the critical C6-position, it should be responsible for the dichotomy in the reactivity of methyl and ethyl acetone-1,3-dicarboxylates. Indeed, as shown in Fig.1, the hydroxy oxygen of the 2-hydroxyphenyl derivative of (1) would have an unrestricted access to C6 and hence the oxygen-bridged pyrimidine (3) would easily be formed. By contrast, due to the different geometry of the ester-methylene grouping in (2), the bulky ethoxy moiety points towards the heterocycle where it sterically hinders the nucleophilic addition of the ortho-hydroxyl on the pyrimidine C5=C6 double bond, with the net result being the 'open', uncyclized molecule (2).
The crystal packing is governed by hydrogen bonding. As shown in Table 2, there are two independent hydrogen bonds, one joins the molecules into dimers and the other links the dimers into chains.
Experimental
Synthesis of the title compound, (1), has been described (Světlík et al., 2008). In short, heating of benzaldehyde (0.51 ml, 5 mmol) with dimethyl acetone-1,3-dicarboxylate (0.73 ml, 5 mmol) and urea (0.36 g, 6 mmol) under p-toluenesulfonic acid (0.04 g, 0.2 mmol) catalysis without solvent at 353–363 K for 3 h gave desired product (39% yield; m.p. 453–455 K). Crystals suitable for the X-ray analysis were obtained by a slow crystallization from ethanol.
Refinement
H atoms were visible in difference maps and were subsequently treated as riding atoms with distances C—H = 0.93 Å (CHarom), 0.97 (CH2) or 0.98 Å (CH), 0.96 Å (CH3) and N—H = 0.86 Å; Uiso of the H atoms were set to 1.2 (1.5 for the methyl H atoms) times Ueq of the parent atom. Because of the indication from Hirshfeld test (Hirshfeld, 1976), 54 rigid-bond restraints on anisotropic displacement parameters for all bonds involving the non-H atoms were applied during the least-squares refinement. Reflection 110, affected by secondary extinction, was deleted from the refinement.
Figures
Fig. 1.
Displacement ellipsoid plot of (1) with the labelling scheme for the non-H atoms, which are drawn as 35% probability ellipsoids.
Fig. 2.
The structures of (1), (2) and (3).
Crystal data
| C15H16N2O5 | F000 = 1280 |
| Mr = 304.35 | Dx = 1.320 Mg m−3 |
| Monoclinic, C2/c | Melting point: 454 K |
| Hall symbol: -C 2yc | Mo Kα radiation λ = 0.71073 Å |
| a = 23.498 (5) Å | Cell parameters from 20 reflections |
| b = 12.072 (2) Å | θ = 7–18º |
| c = 10.933 (5) Å | µ = 0.10 mm−1 |
| β = 99.15 (2)º | T = 296 (2) K |
| V = 3061.9 (16) Å3 | Prism, colourless |
| Z = 8 | 0.30 × 0.25 × 0.20 mm |
Data collection
| Siemens P4 diffractometer | Rint = 0.040 |
| Radiation source: fine-focus sealed tube | θmax = 30.0º |
| Monochromator: graphite | θmin = 1.9º |
| T = 296(2) K | h = −1→32 |
| ω/2θ scans | k = −1→16 |
| Absorption correction: none | l = −15→15 |
| 5187 measured reflections | 3 standard reflections |
| 4466 independent reflections | every 97 reflections |
| 2308 reflections with I > 2σ(I) | intensity decay: none |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.046 | H-atom parameters constrained |
| wR(F2) = 0.118 | w = 1/[σ2(Fo2) + (0.0575P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.90 | (Δ/σ)max = 0.001 |
| 4466 reflections | Δρmax = 0.13 e Å−3 |
| 201 parameters | Δρmin = −0.27 e Å−3 |
| 54 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.44833 (5) | 0.44128 (9) | 0.38217 (11) | 0.0509 (3) | |
| H1 | 0.4615 | 0.4278 | 0.4587 | 0.061* | |
| C2 | 0.44596 (5) | 0.55052 (11) | 0.34279 (13) | 0.0477 (3) | |
| O1 | 0.47167 (4) | 0.62359 (8) | 0.40833 (10) | 0.0628 (3) | |
| N3 | 0.41662 (5) | 0.56699 (9) | 0.23011 (11) | 0.0536 (3) | |
| H3 | 0.4214 | 0.6292 | 0.1948 | 0.064* | |
| C4 | 0.37661 (5) | 0.48718 (11) | 0.16121 (13) | 0.0479 (3) | |
| H4 | 0.3779 | 0.4979 | 0.0728 | 0.058* | |
| C5 | 0.39764 (5) | 0.37090 (11) | 0.19483 (12) | 0.0458 (3) | |
| C6 | 0.43076 (5) | 0.35337 (10) | 0.30560 (12) | 0.0444 (3) | |
| C7 | 0.31513 (5) | 0.50992 (12) | 0.18200 (12) | 0.0473 (3) | |
| C8 | 0.28364 (7) | 0.59098 (14) | 0.11197 (16) | 0.0676 (4) | |
| H8 | 0.2998 | 0.6291 | 0.0520 | 0.081* | |
| C9 | 0.22800 (7) | 0.61558 (16) | 0.13099 (19) | 0.0827 (5) | |
| H9 | 0.2069 | 0.6696 | 0.0827 | 0.099* | |
| C10 | 0.20409 (7) | 0.56203 (17) | 0.21879 (18) | 0.0825 (5) | |
| H10 | 0.1669 | 0.5795 | 0.2314 | 0.099* | |
| C11 | 0.23487 (7) | 0.48198 (19) | 0.28903 (18) | 0.0894 (6) | |
| H11 | 0.2185 | 0.4444 | 0.3491 | 0.107* | |
| C12 | 0.29047 (6) | 0.45678 (16) | 0.27056 (15) | 0.0714 (5) | |
| H12 | 0.3113 | 0.4028 | 0.3193 | 0.086* | |
| C13 | 0.37881 (6) | 0.28141 (13) | 0.10727 (13) | 0.0535 (3) | |
| O2 | 0.39408 (5) | 0.18597 (10) | 0.11528 (11) | 0.0759 (4) | |
| O3 | 0.34141 (5) | 0.31856 (10) | 0.01108 (10) | 0.0760 (4) | |
| C14 | 0.32059 (10) | 0.23753 (17) | −0.08185 (17) | 0.1000 (7) | |
| H14A | 0.3526 | 0.2055 | −0.1139 | 0.150* | |
| H14B | 0.2951 | 0.2726 | −0.1480 | 0.150* | |
| H14C | 0.3001 | 0.1805 | −0.0457 | 0.150* | |
| C15 | 0.45308 (6) | 0.24370 (11) | 0.35961 (13) | 0.0513 (3) | |
| H15A | 0.4440 | 0.1867 | 0.2971 | 0.062* | |
| H15B | 0.4947 | 0.2478 | 0.3805 | 0.062* | |
| C16 | 0.42908 (6) | 0.21132 (11) | 0.47094 (14) | 0.0522 (3) | |
| O4 | 0.39511 (5) | 0.26165 (9) | 0.51997 (10) | 0.0646 (3) | |
| O5 | 0.45077 (6) | 0.11392 (10) | 0.51199 (14) | 0.0996 (5) | |
| C17 | 0.43033 (14) | 0.0714 (2) | 0.6208 (3) | 0.1504 (12) | |
| H17A | 0.4401 | 0.1224 | 0.6882 | 0.226* | |
| H17B | 0.4481 | 0.0011 | 0.6430 | 0.226* | |
| H17C | 0.3892 | 0.0623 | 0.6035 | 0.226* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0531 (6) | 0.0410 (5) | 0.0532 (6) | −0.0021 (5) | −0.0084 (5) | 0.0027 (5) |
| C2 | 0.0392 (6) | 0.0429 (7) | 0.0574 (8) | −0.0001 (6) | −0.0032 (6) | 0.0048 (6) |
| O1 | 0.0607 (6) | 0.0434 (5) | 0.0749 (7) | −0.0039 (5) | −0.0177 (5) | −0.0015 (5) |
| N3 | 0.0486 (6) | 0.0447 (6) | 0.0619 (7) | −0.0066 (5) | −0.0078 (5) | 0.0122 (5) |
| C4 | 0.0415 (6) | 0.0464 (7) | 0.0527 (8) | −0.0022 (5) | −0.0022 (5) | 0.0035 (6) |
| C5 | 0.0368 (6) | 0.0455 (6) | 0.0530 (7) | 0.0002 (5) | 0.0010 (5) | 0.0003 (5) |
| C6 | 0.0360 (6) | 0.0407 (6) | 0.0548 (7) | 0.0011 (5) | 0.0017 (5) | 0.0003 (5) |
| C7 | 0.0390 (6) | 0.0490 (7) | 0.0490 (7) | 0.0024 (5) | −0.0080 (5) | −0.0046 (6) |
| C8 | 0.0557 (8) | 0.0689 (11) | 0.0743 (10) | 0.0115 (7) | −0.0019 (7) | 0.0143 (8) |
| C9 | 0.0596 (9) | 0.0825 (12) | 0.0996 (14) | 0.0289 (9) | −0.0070 (8) | 0.0080 (10) |
| C10 | 0.0446 (8) | 0.1046 (15) | 0.0955 (13) | 0.0202 (9) | 0.0028 (8) | −0.0154 (10) |
| C11 | 0.0569 (9) | 0.1245 (17) | 0.0893 (13) | 0.0169 (10) | 0.0190 (9) | 0.0189 (11) |
| C12 | 0.0502 (8) | 0.0883 (12) | 0.0746 (11) | 0.0138 (8) | 0.0064 (7) | 0.0244 (9) |
| C13 | 0.0462 (7) | 0.0525 (8) | 0.0594 (8) | −0.0017 (6) | 0.0006 (6) | −0.0029 (6) |
| O2 | 0.0868 (8) | 0.0517 (6) | 0.0811 (8) | 0.0034 (6) | −0.0118 (6) | −0.0112 (5) |
| O3 | 0.0810 (8) | 0.0686 (7) | 0.0670 (7) | 0.0045 (6) | −0.0236 (6) | −0.0132 (5) |
| C14 | 0.1211 (17) | 0.0911 (14) | 0.0731 (11) | −0.0022 (13) | −0.0300 (11) | −0.0234 (10) |
| C15 | 0.0430 (7) | 0.0420 (7) | 0.0655 (8) | 0.0065 (6) | −0.0020 (6) | 0.0002 (6) |
| C16 | 0.0445 (7) | 0.0347 (7) | 0.0735 (9) | −0.0071 (6) | −0.0023 (6) | 0.0033 (6) |
| O4 | 0.0629 (6) | 0.0556 (6) | 0.0764 (7) | −0.0047 (5) | 0.0143 (5) | 0.0000 (5) |
| O5 | 0.1040 (10) | 0.0554 (7) | 0.1434 (12) | 0.0181 (7) | 0.0316 (9) | 0.0462 (8) |
| C17 | 0.172 (3) | 0.1040 (19) | 0.187 (3) | 0.0252 (18) | 0.065 (2) | 0.0924 (19) |
Geometric parameters (Å, °)
| N1—C6 | 1.3739 (16) | C10—H10 | 0.9300 |
| N1—C2 | 1.3856 (17) | C11—C12 | 1.387 (2) |
| N1—H1 | 0.8600 | C11—H11 | 0.9300 |
| C2—O1 | 1.2324 (16) | C12—H12 | 0.9300 |
| C2—N3 | 1.3276 (17) | C13—O2 | 1.2058 (18) |
| N3—C4 | 1.4677 (16) | C13—O3 | 1.3362 (17) |
| N3—H3 | 0.8600 | O3—C14 | 1.4388 (19) |
| C4—C5 | 1.5139 (19) | C14—H14A | 0.9600 |
| C4—C7 | 1.5228 (18) | C14—H14B | 0.9600 |
| C4—H4 | 0.9800 | C14—H14C | 0.9600 |
| C5—C6 | 1.3480 (19) | C15—C16 | 1.473 (2) |
| C5—C13 | 1.464 (2) | C15—H15A | 0.9700 |
| C6—C15 | 1.5093 (17) | C15—H15B | 0.9700 |
| C7—C12 | 1.365 (2) | C16—O4 | 1.1957 (17) |
| C7—C8 | 1.3824 (19) | C16—O5 | 1.3308 (17) |
| C8—C9 | 1.388 (2) | O5—C17 | 1.446 (3) |
| C8—H8 | 0.9300 | C17—H17A | 0.9600 |
| C9—C10 | 1.352 (3) | C17—H17B | 0.9600 |
| C9—H9 | 0.9300 | C17—H17C | 0.9600 |
| C10—C11 | 1.368 (3) | ||
| C6—N1—C2 | 123.54 (11) | C10—C11—C12 | 119.97 (19) |
| C6—N1—H1 | 118.2 | C10—C11—H11 | 120.0 |
| C2—N1—H1 | 118.2 | C12—C11—H11 | 120.0 |
| O1—C2—N3 | 124.51 (13) | C7—C12—C11 | 121.07 (16) |
| O1—C2—N1 | 120.59 (12) | C7—C12—H12 | 119.5 |
| N3—C2—N1 | 114.84 (12) | C11—C12—H12 | 119.5 |
| C2—N3—C4 | 125.00 (11) | O2—C13—O3 | 121.92 (14) |
| C2—N3—H3 | 117.5 | O2—C13—C5 | 127.01 (14) |
| C4—N3—H3 | 117.5 | O3—C13—C5 | 111.07 (13) |
| N3—C4—C5 | 109.05 (10) | C13—O3—C14 | 115.78 (13) |
| N3—C4—C7 | 110.52 (11) | O3—C14—H14A | 109.5 |
| C5—C4—C7 | 114.30 (11) | O3—C14—H14B | 109.5 |
| N3—C4—H4 | 107.6 | H14A—C14—H14B | 109.5 |
| C5—C4—H4 | 107.6 | O3—C14—H14C | 109.5 |
| C7—C4—H4 | 107.6 | H14A—C14—H14C | 109.5 |
| C6—C5—C13 | 122.87 (12) | H14B—C14—H14C | 109.5 |
| C6—C5—C4 | 118.89 (11) | C16—C15—C6 | 113.64 (12) |
| C13—C5—C4 | 118.18 (11) | C16—C15—H15A | 108.8 |
| C5—C6—N1 | 120.05 (12) | C6—C15—H15A | 108.8 |
| C5—C6—C15 | 127.18 (12) | C16—C15—H15B | 108.8 |
| N1—C6—C15 | 112.77 (11) | C6—C15—H15B | 108.8 |
| C12—C7—C8 | 118.39 (14) | H15A—C15—H15B | 107.7 |
| C12—C7—C4 | 122.75 (12) | O4—C16—O5 | 123.03 (15) |
| C8—C7—C4 | 118.80 (13) | O4—C16—C15 | 127.31 (13) |
| C7—C8—C9 | 120.14 (17) | O5—C16—C15 | 109.66 (14) |
| C7—C8—H8 | 119.9 | C16—O5—C17 | 115.58 (17) |
| C9—C8—H8 | 119.9 | O5—C17—H17A | 109.5 |
| C10—C9—C8 | 120.80 (17) | O5—C17—H17B | 109.5 |
| C10—C9—H9 | 119.6 | H17A—C17—H17B | 109.5 |
| C8—C9—H9 | 119.6 | O5—C17—H17C | 109.5 |
| C9—C10—C11 | 119.62 (17) | H17A—C17—H17C | 109.5 |
| C9—C10—H10 | 120.2 | H17B—C17—H17C | 109.5 |
| C11—C10—H10 | 120.2 | ||
| C6—N1—C2—O1 | −166.61 (13) | C12—C7—C8—C9 | −1.0 (2) |
| C6—N1—C2—N3 | 10.7 (2) | C4—C7—C8—C9 | −178.35 (15) |
| O1—C2—N3—C4 | −167.13 (13) | C7—C8—C9—C10 | 0.8 (3) |
| N1—C2—N3—C4 | 15.7 (2) | C8—C9—C10—C11 | −0.6 (3) |
| C2—N3—C4—C5 | −32.53 (18) | C9—C10—C11—C12 | 0.5 (3) |
| C2—N3—C4—C7 | 93.93 (15) | C8—C7—C12—C11 | 1.0 (3) |
| N3—C4—C5—C6 | 25.51 (17) | C4—C7—C12—C11 | 178.18 (16) |
| C7—C4—C5—C6 | −98.74 (14) | C10—C11—C12—C7 | −0.7 (3) |
| N3—C4—C5—C13 | −157.16 (12) | C6—C5—C13—O2 | −7.8 (2) |
| C7—C4—C5—C13 | 78.58 (16) | C4—C5—C13—O2 | 174.99 (14) |
| C13—C5—C6—N1 | 178.17 (13) | C6—C5—C13—O3 | 173.11 (13) |
| C4—C5—C6—N1 | −4.6 (2) | C4—C5—C13—O3 | −4.10 (18) |
| C13—C5—C6—C15 | −1.0 (2) | O2—C13—O3—C14 | 0.1 (2) |
| C4—C5—C6—C15 | 176.17 (12) | C5—C13—O3—C14 | 179.23 (16) |
| C2—N1—C6—C5 | −15.8 (2) | C5—C6—C15—C16 | −114.21 (16) |
| C2—N1—C6—C15 | 163.47 (12) | N1—C6—C15—C16 | 66.54 (15) |
| N3—C4—C7—C12 | −94.68 (17) | C6—C15—C16—O4 | 0.0 (2) |
| C5—C4—C7—C12 | 28.79 (19) | C6—C15—C16—O5 | 179.74 (12) |
| N3—C4—C7—C8 | 82.53 (15) | O4—C16—O5—C17 | 0.3 (3) |
| C5—C4—C7—C8 | −154.00 (13) | C15—C16—O5—C17 | −179.39 (18) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1i | 0.86 | 2.06 | 2.8326 (17) | 149 |
| N3—H3···O4ii | 0.86 | 2.32 | 3.0730 (17) | 146 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x, −y+1, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BV2102).
References
- Haggarty, S. J., Mayer, T. U., Miyamoto, D. T., Fathi, R., King, R. W., Mitchison, T. J. & Schreiber, S. L. (2000). Chem. Biol.7, 275–286. [DOI] [PubMed]
- Hirshfeld, F. L. (1976). Acta Cryst. A32, 239–244.
- Kettmann, V., Světlík, J. & Veizerová, L. (2008). Acta Cryst. E64, o1092. [DOI] [PMC free article] [PubMed]
- Klein, E., DeBonis, S., Thiede, B., Skoufias, D. A., Kozielski, F. & Lebeau, L. (2007). Bioorg. Med. Chem.15, 6474–6488. [DOI] [PubMed]
- Mayer, T. U., Kapoor, T. M., Haggarty, S. J., King, R. W., King, R. W., Schreiber, S. L. & Mitchison, T. J. (1999). Science, 286, 971–974. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1991). XSCANS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Světlík, J., Veizerová, L. & Kettmann, V. (2008). Tetrahedron Lett.49, 3520–3523.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808026135/bv2102sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808026135/bv2102Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




