Abstract
The molecule of the title compound, C18H14N4, lies on a center of inversion such that there is one half-molecule in the asymmetric unit. The N—N single bond adopts a trans configuration and the indole fused-ring system is nearly coplanar with the –CH=N—N=CH– fragment [dihedral angle = 9.8 (2)°]. Adjacent molecules are linked by indole–azine N—H⋯N hydrogen bonds into a layer motif.
Related literature
For the synthesis, see: Alemany et al. (1970 ▶); Swaminathan & Narasimhan (1964 ▶). For the crystal structures of some aromatic azines, for example, benzalazine, see: Burke-Laing & Laing (1976 ▶); Mom & de With (1978 ▶); Sinha, 1970 ▶). For other heterocyclic aldehyde azines, see: Lin et al. (2001a
▶,b
▶); Wu et al. (2006 ▶).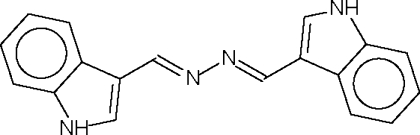
Experimental
Crystal data
C18H14N4
M r = 286.33
Monoclinic,

a = 5.0849 (2) Å
b = 10.6708 (4) Å
c = 13.4435 (5) Å
β = 94.366 (3)°
V = 727.33 (5) Å3
Z = 2
Mo Kα radiation
μ = 0.08 mm−1
T = 295 (2) K
0.33 × 0.27 × 0.17 mm
Data collection
Bruker APEX2 diffractometer
Absorption correction: none
5388 measured reflections
1659 independent reflections
1085 reflections with I > 2σ(I)
R int = 0.038
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.121
S = 1.01
1659 reflections
105 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.17 e Å−3
Δρmin = −0.16 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808003164/fl2186sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808003164/fl2186Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯N2i | 0.87 (2) | 2.21 (2) | 3.065 (2) | 167 (2) |
Symmetry code: (i)  .
.
Acknowledgments
We thank the Science Fund (12–02-03–2031) for supporting this study, and the University of Malaya for the purchase of the diffractometer.
supplementary crystallographic information
Comment
Azines are readily synthesized by condensing hydrazine with an aldehyde; the crystal structures of a large number of substituted benzaldehdye azines have been reported. The structure of the parent aromatic compound, benzalazine, has been known for a long time (Burke-Laing & Laing, 1976; Mom & de With, 1978; Sinha, 1970). There are few examples of heterocyclic azines, and their rarity can be attributed to the difficulty of synthesizing the starting aldehyde reactant. Among the few are, for example, unsubstituted and methyl-subsituted thiophene-2-aldehyde azine (Lin et al., 2001a, 2001b) and a pyrrole derivative has recently been reported (Wu et al., 2006).
3-Indole azine has been known for some time; it was first synthesized from indole-3-carboxaldehyde and hydrazine in order to examine its psychopharmacological activity (Alemany et al., 1970; Swaminathan Narasimhan, 1964). The title compound was the unexpected decomposition product of the Schiff base derived from the condensation of carbohydrazide and indole-3-carboxaldehyde. The molecule (Scheme I, Fig. 1) lies about a center-of-inversion such that there is half a molecule in the asymmetric unit. The N–N single-bond adopts a trans configuration and the indolyl fused-ring is nearly coplanar with the –CH=N–N=CH– fragment. Adjacent molecules are linked by an N–Hindole···Nazine hydrogen bonds into layer motif (Fig. 2).
Experimental
The reaction of carbohydrazide (0.3 g, 3.3 mmol) and indole -3-carboxaldehyde (1 g, 6.6 mmol) in ethanol under reflux for 2 h gave the corresponding Schiff base. This compound (0.2 g, 0.6 mmol), zinc acetate (0.06 g,0.3 mmol) and several drops of triethylamine were dissolved in 10 ml e thanol. The contents were heated in a 25-ml, stainless-steel Paar bomb for for 2 d at 373 K. The bomb was cooled to room temperature over several hours. Well formed crystals were isolated from the cooled bomb.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2U(C). The amino H-atom was located in a difference Fourier map, and was freely refined.
Figures
Fig. 1.
Displacement ellipsoid plot of (I) at the 50% probability level. H atoms are drawn as spheres of arbitrary radiius.
Fig. 2.
Layer structure of (I).
Crystal data
| C18H14N4 | F000 = 300 |
| Mr = 286.33 | Dx = 1.307 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 1012 reflections |
| a = 5.0849 (2) Å | θ = 2.3–23.6º |
| b = 10.6708 (4) Å | µ = 0.08 mm−1 |
| c = 13.4435 (5) Å | T = 295 (2) K |
| β = 94.366 (3)º | Irregular block, green–yellow |
| V = 727.33 (5) Å3 | 0.33 × 0.27 × 0.17 mm |
| Z = 2 |
Data collection
| Bruker APEX2 diffractometer | 1085 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.038 |
| Monochromator: graphite | θmax = 27.5º |
| T = 295(2) K | θmin = 2.4º |
| φ and ω scans | h = −6→6 |
| Absorption correction: none | k = −9→13 |
| 5388 measured reflections | l = −17→17 |
| 1659 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.042 | w = 1/[σ2(Fo2) + (0.0645P)2 + ] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.121 | (Δ/σ)max = 0.001 |
| S = 1.01 | Δρmax = 0.17 e Å−3 |
| 1659 reflections | Δρmin = −0.16 e Å−3 |
| 105 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.016 (6) |
| Secondary atom site location: difference Fourier map |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.4730 (3) | 0.8059 (1) | 0.1978 (1) | 0.0484 (4) | |
| N2 | 0.4464 (2) | 0.5165 (1) | 0.4519 (1) | 0.0422 (4) | |
| C1 | 0.2718 (3) | 0.7222 (1) | 0.1726 (1) | 0.0425 (4) | |
| C2 | 0.1064 (3) | 0.7130 (2) | 0.0860 (1) | 0.0538 (5) | |
| C3 | −0.0787 (3) | 0.6201 (2) | 0.0817 (1) | 0.0579 (5) | |
| C4 | −0.1034 (4) | 0.5384 (2) | 0.1612 (1) | 0.0546 (4) | |
| C5 | 0.0606 (3) | 0.5473 (1) | 0.2473 (1) | 0.0454 (4) | |
| C6 | 0.2548 (3) | 0.6396 (1) | 0.2540 (1) | 0.0388 (4) | |
| C7 | 0.4578 (3) | 0.6773 (1) | 0.3285 (1) | 0.0400 (4) | |
| C8 | 0.5819 (3) | 0.7783 (1) | 0.2901 (1) | 0.0466 (4) | |
| C9 | 0.5376 (3) | 0.6213 (1) | 0.4228 (1) | 0.0411 (4) | |
| H1 | 0.524 (3) | 0.865 (2) | 0.159 (1) | 0.062 (5)* | |
| H2 | 0.1210 | 0.7680 | 0.0330 | 0.065* | |
| H3 | −0.1911 | 0.6111 | 0.0243 | 0.070* | |
| H4 | −0.2329 | 0.4767 | 0.1561 | 0.065* | |
| H5 | 0.0419 | 0.4926 | 0.3002 | 0.054* | |
| H8 | 0.7215 | 0.8217 | 0.3228 | 0.056* | |
| H9 | 0.6619 | 0.6629 | 0.4650 | 0.049* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.061 (1) | 0.041 (1) | 0.043 (1) | −0.002 (1) | 0.005 (1) | 0.012 (1) |
| N2 | 0.063 (1) | 0.036 (1) | 0.027 (1) | −0.001 (1) | −0.003 (1) | 0.001 (1) |
| C1 | 0.049 (1) | 0.039 (1) | 0.039 (1) | 0.006 (1) | 0.006 (1) | 0.005 (1) |
| C2 | 0.062 (1) | 0.058 (1) | 0.040 (1) | 0.007 (1) | −0.001 (1) | 0.014 (1) |
| C3 | 0.060 (1) | 0.065 (1) | 0.047 (1) | 0.005 (1) | −0.008 (1) | 0.003 (1) |
| C4 | 0.054 (1) | 0.050 (1) | 0.059 (1) | −0.003 (1) | −0.001 (1) | 0.000 (1) |
| C5 | 0.052 (1) | 0.040 (1) | 0.045 (1) | 0.003 (1) | 0.006 (1) | 0.004 (1) |
| C6 | 0.046 (1) | 0.035 (1) | 0.036 (1) | 0.007 (1) | 0.007 (1) | 0.003 (1) |
| C7 | 0.052 (1) | 0.034 (1) | 0.034 (1) | 0.004 (1) | 0.004 (1) | 0.001 (1) |
| C8 | 0.059 (1) | 0.040 (1) | 0.041 (1) | −0.002 (1) | 0.001 (1) | 0.003 (1) |
| C9 | 0.056 (1) | 0.036 (1) | 0.032 (1) | −0.003 (1) | −0.001 (1) | −0.003 (1) |
Geometric parameters (Å, °)
| N1—C8 | 1.351 (2) | C6—C7 | 1.440 (2) |
| N1—C1 | 1.381 (2) | C7—C8 | 1.370 (2) |
| N2—C9 | 1.283 (2) | C7—C9 | 1.432 (2) |
| N2—N2i | 1.409 (2) | N1—H1 | 0.87 (2) |
| C1—C2 | 1.387 (2) | C2—H2 | 0.9300 |
| C1—C6 | 1.412 (2) | C3—H3 | 0.9300 |
| C2—C3 | 1.365 (2) | C4—H4 | 0.9300 |
| C3—C4 | 1.393 (2) | C5—H5 | 0.9300 |
| C4—C5 | 1.377 (2) | C8—H8 | 0.9300 |
| C5—C6 | 1.393 (2) | C9—H9 | 0.9300 |
| C8—N1—C1 | 109.2 (1) | N2—C9—C7 | 123.4 (1) |
| C9—N2—N2i | 112.0 (1) | C8—N1—H1 | 126 (1) |
| N1—C1—C2 | 129.9 (1) | C1—N1—H1 | 125 (1) |
| N1—C1—C6 | 107.6 (1) | C3—C2—H2 | 121.4 |
| C2—C1—C6 | 122.5 (2) | C1—C2—H2 | 121.4 |
| C3—C2—C1 | 117.3 (2) | C2—C3—H3 | 119.2 |
| C2—C3—C4 | 121.7 (2) | C4—C3—H3 | 119.2 |
| C5—C4—C3 | 121.2 (2) | C5—C4—H4 | 119.4 |
| C4—C5—C6 | 118.9 (1) | C3—C4—H4 | 119.4 |
| C5—C6—C1 | 118.5 (1) | C4—C5—H5 | 120.5 |
| C5—C6—C7 | 135.2 (1) | C6—C5—H5 | 120.5 |
| C1—C6—C7 | 106.3 (1) | N1—C8—H8 | 124.8 |
| C8—C7—C9 | 123.7 (1) | C7—C8—H8 | 124.8 |
| C8—C7—C6 | 106.5 (1) | N2—C9—H9 | 118.3 |
| C9—C7—C6 | 129.7 (1) | C7—C9—H9 | 118.3 |
| N1—C8—C7 | 110.5 (1) | ||
| C8—N1—C1—C2 | −179.5 (2) | C2—C1—C6—C7 | 179.4 (1) |
| C8—N1—C1—C6 | 0.5 (2) | C5—C6—C7—C8 | −178.6 (2) |
| N1—C1—C2—C3 | −179.6 (2) | C1—C6—C7—C8 | 0.5 (2) |
| C6—C1—C2—C3 | 0.4 (2) | C5—C6—C7—C9 | 5.1 (3) |
| C1—C2—C3—C4 | 0.7 (3) | C1—C6—C7—C9 | −175.9 (2) |
| C2—C3—C4—C5 | −0.7 (3) | C1—N1—C8—C7 | −0.2 (2) |
| C3—C4—C5—C6 | −0.3 (2) | C9—C7—C8—N1 | 176.5 (1) |
| C4—C5—C6—C1 | 1.3 (2) | C6—C7—C8—N1 | −0.2 (2) |
| C4—C5—C6—C7 | −179.7 (2) | N2i—N2—C9—C7 | −178.9 (1) |
| N1—C1—C6—C5 | 178.6 (1) | C8—C7—C9—N2 | −169.5 (1) |
| C2—C1—C6—C5 | −1.4 (2) | C6—C7—C9—N2 | 6.3 (3) |
| N1—C1—C6—C7 | −0.6 (2) |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···N2ii | 0.87 (2) | 2.21 (2) | 3.065 (2) | 167 (2) |
Symmetry codes: (ii) −x+1, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FL2186).
References
- Alemany, A., Bernabe, M., Fernandez Alvarez, E., Lora-Tamayo, M. & Nieto Lopez, O. (1970). Anal. Quim.66, 681–688.
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Burke-Laing, M. & Laing, M. (1976). Acta Cryst. B32, 3216–3224.
- Lin, C.-J., Hwang, W.-S. & Chiang, M. J. (2001a). J. Organomet. Chem.640, 85–92.
- Lin, C.-J., Hwang, W.-S. & Chiang, M. J. (2001b). Polyhedron, 20, 3257–3264.
- Mom, V. & de With, G. (1978). Acta Cryst. B34, 2785–2789.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sinha, U. C. (1970). Acta Cryst. B26, 889–895.
- Swaminathan, S. & Narasimhan, K. (1964). Indian J. Chem.2, 423–424.
- Westrip, S. P. (2008). publCIF In preparation.
- Wu, C.-Y., Chen, Y., Jing, S.-Y., Lee, C.-S., Dinda, J. & Hwang, W.-S. (2006). Polyhedron, 25, 3053–3065.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808003164/fl2186sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808003164/fl2186Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




