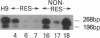Abstract
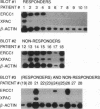
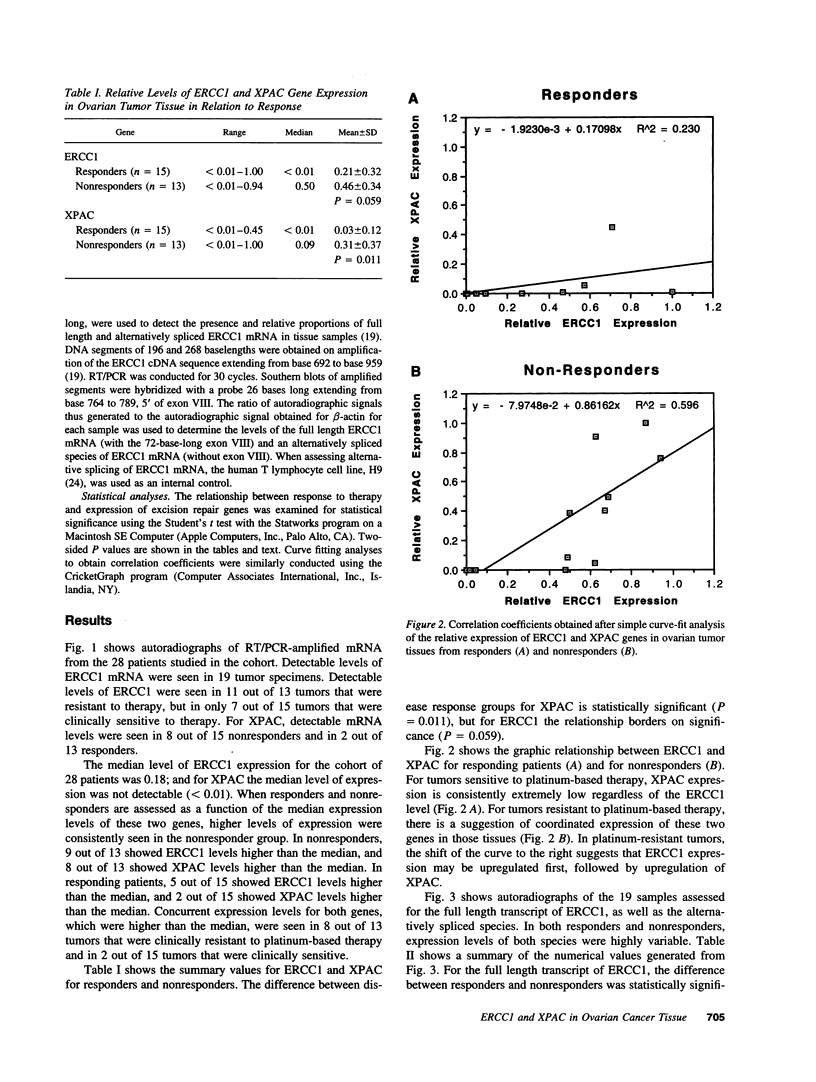
Nucleotide excision repair is a DNA repair pathway that is highly conserved in nature, with analogous repair systems described in Escherichia coli, yeast, and mammalian cells. The rate-limiting step, DNA damage recognition and excision, is effected by the protein products of the genes ERCC1 and XPAC. We therefore assessed mRNA levels of ERCC1 and XPAC in malignant ovarian cancer tissues from 28 patients that were harvested before the administration of platinum-based chemotherapy. Cancer tissues from patients whose tumors were clinically resistant to therapy (n = 13) showed greater levels of total ERCC1 mRNA (P = 0.059), full length transcript of ERCC1 mRNA (P = 0.026), and XPAC mRNA (P = 0.011), as compared with tumor tissues from those individuals clinically sensitive to therapy (n = 15). In 19 of these tissues, the percentage of alternative splicing of ERCC1 mRNA was assessed. ERCC1 splicing was highly variable, with no difference observed between responders and nonresponders. The alternatively spliced species constituted 2-58% of the total ERCC1 mRNA in responders (median = 18%) and 4-71% in nonresponders (median = 13%). These data suggest greater activity of the DNA excision repair genes ERCC1 and XPAC in ovarian cancer tissues of patients clinically resistant to platinum compounds. These data also indicate highly variable splicing of ERCC1 mRNA in ovarian cancer tissues in vivo, whether or not such tissues are sensitive to platinum-based therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankmann M., Prakash L., Prakash S. Yeast RAD14 and human xeroderma pigmentosum group A DNA-repair genes encode homologous proteins. Nature. 1992 Feb 6;355(6360):555–558. doi: 10.1038/355555a0. [DOI] [PubMed] [Google Scholar]
- Belt P. B., van Oosterwijk M. F., Odijk H., Hoeijmakers J. H., Backendorf C. Induction of a mutant phenotype in human repair proficient cells after overexpression of a mutated human DNA repair gene. Nucleic Acids Res. 1991 Oct 25;19(20):5633–5637. doi: 10.1093/nar/19.20.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Berg P. DNA cross-linked by cisplatin: a new probe for the DNA repair defect in xeroderma pigmentosum. Mol Biol Med. 1987 Oct;4(5):277–290. [PubMed] [Google Scholar]
- Cleaver J. E. Do we know the cause of xeroderma pigmentosum? Carcinogenesis. 1990 Jun;11(6):875–882. doi: 10.1093/carcin/11.6.875. [DOI] [PubMed] [Google Scholar]
- Dabholkar M., Bostick-Bruton F., Weber C., Bohr V. A., Egwuagu C., Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992 Oct 7;84(19):1512–1517. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- Dabholkar M., Bostick-Bruton F., Weber C., Egwuagu C., Bohr V. A., Reed E. Expression of excision repair genes in non-malignant bone marrow from cancer patients. Mutat Res. 1993 Jan;293(2):151–160. doi: 10.1016/0921-8777(93)90066-p. [DOI] [PubMed] [Google Scholar]
- Dabholkar M., Parker R., Reed E. Determinants of cisplatin sensitivity in non-malignant non-drug-selected human T cell lines. Mutat Res. 1992 Jun;274(1):45–56. doi: 10.1016/0921-8777(92)90042-2. [DOI] [PubMed] [Google Scholar]
- Dijt F. J., Fichtinger-Schepman A. M., Berends F., Reedijk J. Formation and repair of cisplatin-induced adducts to DNA in cultured normal and repair-deficient human fibroblasts. Cancer Res. 1988 Nov 1;48(21):6058–6062. [PubMed] [Google Scholar]
- Geleziunas R., McQuillan A., Malapetsa A., Hutchinson M., Kopriva D., Wainberg M. A., Hiscott J., Bramson J., Panasci L. Increased DNA synthesis and repair-enzyme expression in lymphocytes from patients with chronic lymphocytic leukemia resistant to nitrogen mustards. J Natl Cancer Inst. 1991 Apr 17;83(8):557–564. doi: 10.1093/jnci/83.8.557. [DOI] [PubMed] [Google Scholar]
- Hansson J., Grossman L., Lindahl T., Wood R. D. Complementation of the xeroderma pigmentosum DNA repair synthesis defect with Escherichia coli UvrABC proteins in a cell-free system. Nucleic Acids Res. 1990 Jan 11;18(1):35–40. doi: 10.1093/nar/18.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Hoeijmakers J. H. How relevant is the Escherichia coli UvrABC model for excision repair in eukaryotes? J Cell Sci. 1991 Dec;100(Pt 4):687–691. doi: 10.1242/jcs.100.4.687. [DOI] [PubMed] [Google Scholar]
- Lee K. B., Parker R. J., Bohr V., Cornelison T., Reed E. Cisplatin sensitivity/resistance in UV repair-deficient Chinese hamster ovary cells of complementation groups 1 and 3. Carcinogenesis. 1993 Oct;14(10):2177–2180. doi: 10.1093/carcin/14.10.2177. [DOI] [PubMed] [Google Scholar]
- Miyamoto I., Miura N., Niwa H., Miyazaki J., Tanaka K. Mutational analysis of the structure and function of the xeroderma pigmentosum group A complementing protein. Identification of essential domains for nuclear localization and DNA excision repair. J Biol Chem. 1992 Jun 15;267(17):12182–12187. [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. J., Eastman A., Bostick-Bruton F., Reed E. Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin-DNA lesions and reduced drug accumulation. J Clin Invest. 1991 Mar;87(3):772–777. doi: 10.1172/JCI115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll E. H., Abrahams P. J., Arwert F., Eriksson A. W. Host-cell reactivation of cis-diamminedichloroplatinum(II)-treated SV40 DNA in normal human, Fanconi anaemia and xeroderma pigmentosum fibroblasts. Mutat Res. 1984 Nov-Dec;132(5-6):181–187. doi: 10.1016/0167-8817(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Popovic M., Read-Connole E., Gallo R. C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet. 1984 Dec 22;2(8417-8418):1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- Reed E., Janik J., Bookman M. A., Rothenberg M., Smith J., Young R. C., Ozols R. F., VanderMolen L., Kohn E., Jacob J. L. High-dose carboplatin and recombinant granulocyte-macrophage colony-stimulating factor in advanced-stage recurrent ovarian cancer. J Clin Oncol. 1993 Nov;11(11):2118–2126. doi: 10.1200/JCO.1993.11.11.2118. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. L., Ostchega Y., Steinberg S. M., Young R. C., Hummel S., Ozols R. F. High-dose carboplatin with diethyldithiocarbamate chemoprotection in treatment of women with relapsed ovarian cancer. J Natl Cancer Inst. 1988 Nov 16;80(18):1488–1492. doi: 10.1093/jnci/80.18.1488. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. L., Ozols R. F., Glatstein E., Steinberg S. M., Reed E., Young R. C. Dose-intensive induction therapy with cyclophosphamide, cisplatin, and consolidative abdominal radiation in advanced-stage epithelial ovarian cancer. J Clin Oncol. 1992 May;10(5):727–734. doi: 10.1200/JCO.1992.10.5.727. [DOI] [PubMed] [Google Scholar]
- Satokata I., Tanaka K., Yuba S., Okada Y. Identification of splicing mutations of the last nucleotides of exons, a nonsense mutation, and a missense mutation of the XPAC gene as causes of group A xeroderma pigmentosum. Mutat Res. 1992 Mar;273(2):203–212. doi: 10.1016/0921-8777(92)90081-d. [DOI] [PubMed] [Google Scholar]
- Sheibani N., Jennerwein M. M., Eastman A. DNA repair in cells sensitive and resistant to cis-diamminedichloroplatinum(II): host cell reactivation of damaged plasmid DNA. Biochemistry. 1989 Apr 4;28(7):3120–3124. doi: 10.1021/bi00433a055. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Bardwell A. J., Bardwell L., Tappe N. J., Friedberg E. C. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature. 1993 Apr 29;362(6423):860–862. doi: 10.1038/362860a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wang Z., Wu X., Friedberg E. C. Nucleotide-excision repair of DNA in cell-free extracts of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4907–4911. doi: 10.1073/pnas.90.11.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Lindahl T. Xeroderma pigmentosum. A gene for tumour prevention. Nature. 1990 Nov 1;348(6296):13–14. doi: 10.1038/348013a0. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]