Figure 1.
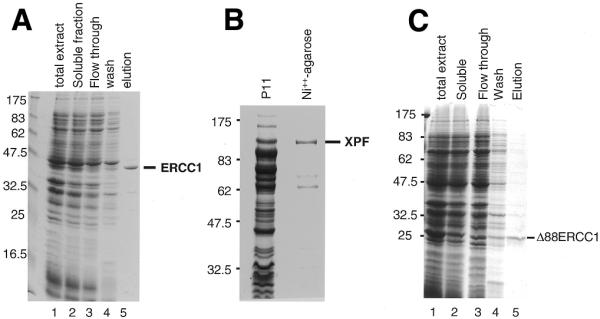
Expression in E.coli and purification of soluble recombinant proteins. (A) Purification of ERCC1 on a Talon Co++ charged resin. Proteins were separated on a 12% SDS–PAGE gel and stained with Coomassie blue. Lane 1, total cellular proteins; lane 2, soluble proteins; lane 3, flow through from a Talon column; lane 4, 10 mM imidazole wash; lane 5, ERCC1 eluted with 500 mM imidazole. Size markers (kDa) are on the left. (B) Purification of XPF. Proteins were separated on an 8% SDS–PAGE gel and stained with Coomassie blue. Sizes (kDa) are shown on the left. The protein lanes show the high salt eluate from P11 phosphocellulose (P11) and the Ni++-agarose eluate. (C) Purification of Δ88ERCC1 on Talon. Proteins were separated on a 12% SDS–PAGE gel and stained with Coomassie blue. Lane 1, total cellular proteins; lane 2, soluble proteins, loaded on Talon; lane 3, flow through; lane 4, 10 mM imidazole wash; lane 5, Δ88ERCC1 eluted with 500 mM imidazole.
